当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structure-Based Design of Non-natural Macrocyclic Peptides That Inhibit Protein–Protein Interactions
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-10-27 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01221 Dennis M. Krüger 1, 2 , Adrian Glas 1, 2 , David Bier 1, 3 , Nicole Pospiech 1 , Kerstin Wallraven 1 , Laura Dietrich 1, 2 , Christian Ottmann 3, 4 , Oliver Koch 2 , Sven Hennig 1, 5 , Tom N. Grossmann 1, 2, 5
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-10-27 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01221 Dennis M. Krüger 1, 2 , Adrian Glas 1, 2 , David Bier 1, 3 , Nicole Pospiech 1 , Kerstin Wallraven 1 , Laura Dietrich 1, 2 , Christian Ottmann 3, 4 , Oliver Koch 2 , Sven Hennig 1, 5 , Tom N. Grossmann 1, 2, 5
Affiliation
|
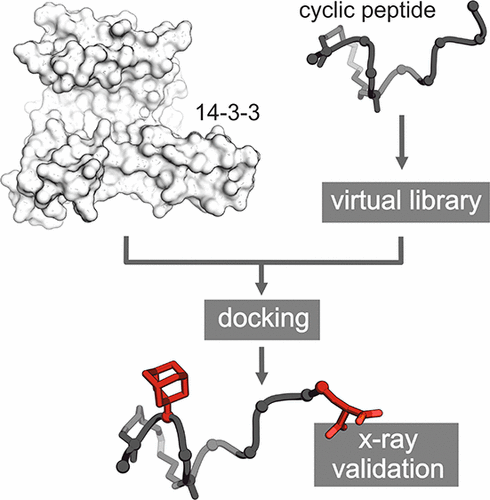
Macrocyclic peptides can interfere with challenging biomolecular targets including protein–protein interactions. Whereas there are various approaches that facilitate the identification of peptide-derived ligands, their evolution into higher affinity binders remains a major hurdle. We report a virtual screen based on molecular docking that allows the affinity maturation of macrocyclic peptides taking non-natural amino acids into consideration. These macrocycles bear large and flexible substituents that usually complicate the use of docking approaches. A virtual library containing more than 1400 structures was screened against the target focusing on docking poses with the core structure resembling a known bioactive conformation. Based on this screen, a macrocyclic peptide 22 involving two non-natural amino acids was evolved showing increased target affinity and biological activity. Predicted binding modes were verified by X-ray crystallography. The presented workflow allows the screening of large macrocyclic peptides with diverse modifications thereby expanding the accessible chemical space and reducing synthetic efforts.
中文翻译:

基于结构的非天然大环肽抑制蛋白质-蛋白质相互作用的设计。
大环肽可以干扰具有挑战性的生物分子靶标,包括蛋白质与蛋白质的相互作用。尽管有多种方法可帮助鉴定肽衍生的配体,但它们向更高亲和力结合剂的进化仍是主要障碍。我们报告了基于分子对接的虚拟屏幕,该屏幕允许考虑非天然氨基酸的大环肽的亲和力成熟。这些大环带有大而灵活的取代基,通常使对接方法的使用复杂化。针对目标筛选了一个包含1400多个结构的虚拟文库,重点是对接姿势,其核心结构类似于已知的生物活性构象。基于此筛选,大环肽22涉及两个非天然氨基酸的进化显示出增加的目标亲和力和生物活性。预测的结合模式通过X射线晶体学验证。提出的工作流程允许筛选具有各种修饰的大型大环肽,从而扩大可及的化学空间并减少合成工作。
更新日期:2017-10-27
中文翻译:

基于结构的非天然大环肽抑制蛋白质-蛋白质相互作用的设计。
大环肽可以干扰具有挑战性的生物分子靶标,包括蛋白质与蛋白质的相互作用。尽管有多种方法可帮助鉴定肽衍生的配体,但它们向更高亲和力结合剂的进化仍是主要障碍。我们报告了基于分子对接的虚拟屏幕,该屏幕允许考虑非天然氨基酸的大环肽的亲和力成熟。这些大环带有大而灵活的取代基,通常使对接方法的使用复杂化。针对目标筛选了一个包含1400多个结构的虚拟文库,重点是对接姿势,其核心结构类似于已知的生物活性构象。基于此筛选,大环肽22涉及两个非天然氨基酸的进化显示出增加的目标亲和力和生物活性。预测的结合模式通过X射线晶体学验证。提出的工作流程允许筛选具有各种修饰的大型大环肽,从而扩大可及的化学空间并减少合成工作。



























 京公网安备 11010802027423号
京公网安备 11010802027423号