当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of a Concise Multikilogram Synthesis of LPA-1 Antagonist BMS-986020 via a Tandem Borylation–Suzuki Procedure
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2017-10-26 00:00:00 , DOI: 10.1021/acs.oprd.7b00301 Michael J. Smith 1 , Michael J. Lawler 1 , Nathaniel Kopp 1 , Douglas D. Mcleod 1 , Akin H. Davulcu 1 , Dong Lin 1 , Kishta Katipally 1 , Chris Sfouggatakis 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2017-10-26 00:00:00 , DOI: 10.1021/acs.oprd.7b00301 Michael J. Smith 1 , Michael J. Lawler 1 , Nathaniel Kopp 1 , Douglas D. Mcleod 1 , Akin H. Davulcu 1 , Dong Lin 1 , Kishta Katipally 1 , Chris Sfouggatakis 1
Affiliation
|
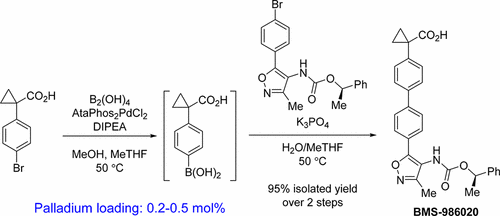
The process development for the synthesis of BMS-986020 (1) via a palladium catalyzed tandem borylation/Suzuki reaction is described. Evaluation of conditions culminated in an efficient borylation procedure using tetrahydroxydiboron followed by a tandem Suzuki reaction employing the same commercially available palladium catalyst for both steps. This methodology addressed shortcomings of early synthetic routes and was ultimately used for the multikilogram scale synthesis of the active pharmaceutical ingredient 1. Further evaluation of the borylation reaction showed useful reactivity with a range of substituted aryl bromides and iodides as coupling partners. These findings represent a practical, efficient, mild, and scalable method for borylation.
中文翻译:

通过串联硼基化-铃木法开发LPA-1拮抗剂BMS-986020的简明多千克合成方法
描述了通过钯催化的串联硼酸化/ Suzuki反应合成BMS-986020(1)的方法。在四步中,使用四羟基二硼进行有效的硼酸酯化步骤,然后进行串联的铃木反应,使用有效的钯化催化剂进行条件评估。该方法论解决了早期合成路线的缺点,并最终用于活性药物成分1的多公斤级合成。对该硼化反应的进一步评估显示出与一系列取代的芳基溴化物和碘化物作为偶联伙伴的有用的反应性。这些发现代表了一种实用,有效,温和且可扩展的硼酸化方法。
更新日期:2017-10-26
中文翻译:

通过串联硼基化-铃木法开发LPA-1拮抗剂BMS-986020的简明多千克合成方法
描述了通过钯催化的串联硼酸化/ Suzuki反应合成BMS-986020(1)的方法。在四步中,使用四羟基二硼进行有效的硼酸酯化步骤,然后进行串联的铃木反应,使用有效的钯化催化剂进行条件评估。该方法论解决了早期合成路线的缺点,并最终用于活性药物成分1的多公斤级合成。对该硼化反应的进一步评估显示出与一系列取代的芳基溴化物和碘化物作为偶联伙伴的有用的反应性。这些发现代表了一种实用,有效,温和且可扩展的硼酸化方法。


























 京公网安备 11010802027423号
京公网安备 11010802027423号