当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Metal-free annulation of β-acylamino ketones: facile access to spirooxazolines and oxazolines via oxidative C–O bond formation
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-10-16 00:00:00 , DOI: 10.1039/c7qo00783c Santosh S. Chavan 1, 2, 3, 4 , Bapurao D. Rupanawar 1, 2, 3, 4 , Rohit B. Kamble 1, 2, 3, 4 , Anil M. Shelke 1, 2, 3, 4 , Gurunath Suryavanshi 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-10-16 00:00:00 , DOI: 10.1039/c7qo00783c Santosh S. Chavan 1, 2, 3, 4 , Bapurao D. Rupanawar 1, 2, 3, 4 , Rohit B. Kamble 1, 2, 3, 4 , Anil M. Shelke 1, 2, 3, 4 , Gurunath Suryavanshi 1, 2, 3, 4
Affiliation
|
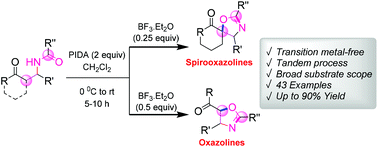
A metal-free annulation reaction of β-acylamino ketone derivatives has been reported for the synthesis of a group of functionalized spirooxazolines and oxazolines in good to excellent yields. The reaction proceeds via phenyliodine(III) diacetate (PIDA)-mediated oxidative C–O bond formation in the presence of BF3–OEt2. The mild reaction conditions, broad substrate scope, simple execution and synthetic potential of the products make this novel protocol very attractive.
中文翻译:

β-酰基氨基酮的无金属环化:通过氧化C–O键的形成容易地获得螺恶唑啉和恶唑啉
已经报道了β-酰基氨基酮衍生物的无金属环化反应,用于合成一组官能化的螺恶唑啉和恶唑啉,收率好至极好。反应在存在BF 3 -OEt 2的情况下通过二乙酸苯基碘(III)(PIDA)介导的氧化C-O键进行。温和的反应条件,广泛的底物范围,简单的操作方法和产品的合成潜力使这种新颖的方案非常有吸引力。
更新日期:2017-10-26
中文翻译:

β-酰基氨基酮的无金属环化:通过氧化C–O键的形成容易地获得螺恶唑啉和恶唑啉
已经报道了β-酰基氨基酮衍生物的无金属环化反应,用于合成一组官能化的螺恶唑啉和恶唑啉,收率好至极好。反应在存在BF 3 -OEt 2的情况下通过二乙酸苯基碘(III)(PIDA)介导的氧化C-O键进行。温和的反应条件,广泛的底物范围,简单的操作方法和产品的合成潜力使这种新颖的方案非常有吸引力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号