当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Active Site Interactions Impact Phosphoryl Transfer during Replication of Damaged and Undamaged DNA by Escherichia coli DNA Polymerase I
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2017-10-25 00:00:00 , DOI: 10.1021/acs.chemrestox.7b00257 A. S. Prakasha Gowda 1 , Thomas E. Spratt 1
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2017-10-25 00:00:00 , DOI: 10.1021/acs.chemrestox.7b00257 A. S. Prakasha Gowda 1 , Thomas E. Spratt 1
Affiliation
|
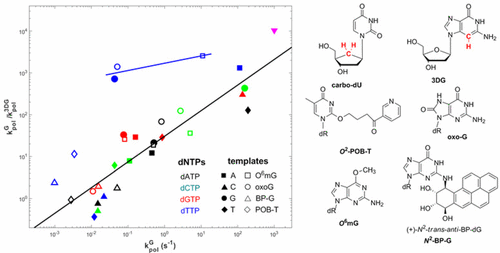
Replicative DNA polymerases are able to discriminate between very similar substrates with high accuracy. One mechanism by which E. coli DNA polymerase I checks for Watson–Crick geometry is through a hydrogen bonding fork between Arg668 and the incoming dNTP and the minor groove of the primer terminus. The importance of the Arg-fork was examined by disrupting it with either a guanine to 3-deazaguanine substitution at the primer terminus or the use of a carbocyclic deoxyribose analog of dUTP. Using thio-substituted dNTPs and differential quench techniques, we determined that when the Arg-fork was disrupted, the rate-limiting step changed from a conformational change to phosphodiester bond formation. This result indicates that Arg668 is involved in the phosphoryl transfer step. We examined the role of the Arg-fork in the replication of four DNA damaged templates, O6-methylguanine (O6-mG), 8-oxo-7,8-dihydroguanine (oxoG), O2-[4-(3-pyridyl)-4-oxobutyl]thymine (O2-POB-T), and N2-[(7S,8R,9S,10R)-7,8,9,10-tetrahydro-8,9,10-trihydroxybenzo[a]pyren-7-yl]-guanine (N2-BP-G). In general, the guanine to 3-deazaguanine substitution caused a decrease in kpol that was proportional to kpol over five orders of magnitude. The linear relationship indicates that the Arg668-fork helps catalyze phosphoryl transfer by the same mechanism with all the substrates. Exceptions to the linear relationship were the incorporations of dTTP opposite G, oxoG, and O6mG, which showed large decreases in kpol, similar to that exhibited by the Watson–Crick base pairs. It was proposed that the incorporation of dTTP opposite G, oxoG, and O6mG occurred via Watson–Crick-like structures.
中文翻译:

活性位点相互作用在大肠杆菌DNA聚合酶I复制受损和未损坏的DNA的过程中影响磷酸转移。
复制性DNA聚合酶能够以很高的准确度区分非常相似的底物。大肠杆菌的一种机制DNA聚合酶I通过Arg668与进入的dNTP和引物末端的小沟之间的氢键叉检查Watson-Crick的几何形状。通过在引物末端用鸟嘌呤取代3-脱氮鸟嘌呤或使用dUTP的碳环脱氧核糖类似物破坏Arg-fork的重要性进行了研究。使用硫取代的dNTPs和差异猝灭技术,我们确定当Arg叉被破坏时,限速步骤从构象变化变为磷酸二酯键形成。该结果表明Arg668参与磷酸基转移步骤。我们研究了Arg叉在四个DNA受损模板O 6-甲基鸟嘌呤(O 6-mG),8-氧代-7,8-二氢鸟嘌呤(oxoG),O 2- [4-(3-吡啶基)-4-氧代丁基]胸腺嘧啶(O 2 -POB-T)和N 2 -[(7 S,8 R,9 S,10 R)-7,8,9,10-四氢-8,9,10-三羟基苯并[ a ] py-7-基]-鸟嘌呤(N 2 -BP -G)。通常,鸟嘌呤到3-脱氮鸟嘌呤置换引起的降低ķ POL这是正比于ķ POL超过五个数量级。线性关系表明,Arg668叉与所有底物的催化机理相同,均有助于催化磷酰基转移。线性关系的例外是与G,oxoG和O 6 mG相对的dTTP的掺入,这表明k pol大大降低,类似于Watson-Crick碱基对所显示的。有人提出,与G,oxoG和O 6 mG相反的dTTP的掺入是通过Watson-Crick样结构发生的。
更新日期:2017-10-26
中文翻译:

活性位点相互作用在大肠杆菌DNA聚合酶I复制受损和未损坏的DNA的过程中影响磷酸转移。
复制性DNA聚合酶能够以很高的准确度区分非常相似的底物。大肠杆菌的一种机制DNA聚合酶I通过Arg668与进入的dNTP和引物末端的小沟之间的氢键叉检查Watson-Crick的几何形状。通过在引物末端用鸟嘌呤取代3-脱氮鸟嘌呤或使用dUTP的碳环脱氧核糖类似物破坏Arg-fork的重要性进行了研究。使用硫取代的dNTPs和差异猝灭技术,我们确定当Arg叉被破坏时,限速步骤从构象变化变为磷酸二酯键形成。该结果表明Arg668参与磷酸基转移步骤。我们研究了Arg叉在四个DNA受损模板O 6-甲基鸟嘌呤(O 6-mG),8-氧代-7,8-二氢鸟嘌呤(oxoG),O 2- [4-(3-吡啶基)-4-氧代丁基]胸腺嘧啶(O 2 -POB-T)和N 2 -[(7 S,8 R,9 S,10 R)-7,8,9,10-四氢-8,9,10-三羟基苯并[ a ] py-7-基]-鸟嘌呤(N 2 -BP -G)。通常,鸟嘌呤到3-脱氮鸟嘌呤置换引起的降低ķ POL这是正比于ķ POL超过五个数量级。线性关系表明,Arg668叉与所有底物的催化机理相同,均有助于催化磷酰基转移。线性关系的例外是与G,oxoG和O 6 mG相对的dTTP的掺入,这表明k pol大大降低,类似于Watson-Crick碱基对所显示的。有人提出,与G,oxoG和O 6 mG相反的dTTP的掺入是通过Watson-Crick样结构发生的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号