当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Highly enantioselective Rh/chiral sulfur-olefin-catalyzed arylation of alkyl-substituted non-benzofused cyclic N-sulfonyl ketimines
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-08-03 00:00:00 , DOI: 10.1039/c7qo00555e Ming-Qing Liu 1, 2, 3, 4, 5 , Tao Jiang 1, 2, 3, 4, 5 , Wen-Wen Chen 1, 2, 3, 4, 5 , Ming-Hua Xu 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-08-03 00:00:00 , DOI: 10.1039/c7qo00555e Ming-Qing Liu 1, 2, 3, 4, 5 , Tao Jiang 1, 2, 3, 4, 5 , Wen-Wen Chen 1, 2, 3, 4, 5 , Ming-Hua Xu 1, 2, 3, 4, 5
Affiliation
|
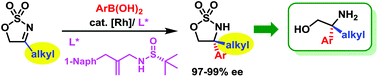
A highly enantioselective Rh-catalyzed addition of arylboronic acids to non-benzofused five-membered cyclic N-sulfonyl alkyl-substituted ketimines has been developed. With simple chiral sulfur-olefin as the ligand, the reaction allows convenient access to a variety of sulfamidates with uniformly excellent enantioselectivities (97–99% ee). The synthetic utility of this protocol was exemplified by rapid asymmetric construction of tetrasubstituted chiral β-amino alcohols and their derivatives.
中文翻译:

烷基取代的非苯并稠合环状N-磺酰基酮亚胺的高对映选择性Rh /手性硫-烯烃催化的芳基化
已经开发了高度对映体的Rh催化的芳基硼酸加成到非苯并稠合的五元环状N-磺酰基烷基取代的酮亚胺上。用简单的手性硫-烯烃作为配体,该反应可方便地获得具有统一优异对映选择性(97-99%ee)的各种氨基磺酸盐。该方案的合成效用以四取代的手性β-氨基醇及其衍生物的快速不对称结构为例。
更新日期:2017-10-25
中文翻译:

烷基取代的非苯并稠合环状N-磺酰基酮亚胺的高对映选择性Rh /手性硫-烯烃催化的芳基化
已经开发了高度对映体的Rh催化的芳基硼酸加成到非苯并稠合的五元环状N-磺酰基烷基取代的酮亚胺上。用简单的手性硫-烯烃作为配体,该反应可方便地获得具有统一优异对映选择性(97-99%ee)的各种氨基磺酸盐。该方案的合成效用以四取代的手性β-氨基醇及其衍生物的快速不对称结构为例。



























 京公网安备 11010802027423号
京公网安备 11010802027423号