当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photoredox meets gold Lewis acid catalysis in the alkylative semipinacol rearrangement: a photocatalyst with a dark side
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-08-01 00:00:00 , DOI: 10.1039/c7qo00590c M. Zidan 1, 2, 3, 4, 5 , T. McCallum 1, 2, 3, 4, 5 , L. Thai-Savard 1, 2, 3, 4, 5 , L. Barriault 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-08-01 00:00:00 , DOI: 10.1039/c7qo00590c M. Zidan 1, 2, 3, 4, 5 , T. McCallum 1, 2, 3, 4, 5 , L. Thai-Savard 1, 2, 3, 4, 5 , L. Barriault 1, 2, 3, 4, 5
Affiliation
|
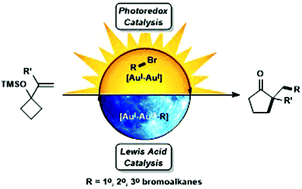
The alkylative semipinacol rearrangement of a variety of TMS protected α-styrenyl substituted cyclic alcohols with unactivated bromoalkanes that merge photoredox and Au(I)/Au(III) catalysis has been achieved. This redox neutral rearrangement is marked by a dimeric Au(I) photocatalyst that plays two roles; photoredox activation of bromoalkanes and Au(III)-mediated semipinacol rearrangement coupled with C(sp3)–C(sp3) reductive elimination; a reaction mode rarely accessed. This operationally simple methodology contains readily available starting materials that undergo reaction with [Au2(dppm)2]Cl2 upon irradiation with UVA LEDs, furnishing diversified ketone products. Primary, secondary, and tertiary bromoalkanes and a range of TMS protected α-styrenyl substituted alcohols were investigated in this transformation.
中文翻译:

Photoredox在烷基半频哪醇重排中遇到金Lewis酸催化:具有深色面的光催化剂
已经实现了多种TMS保护的α-苯乙烯基取代的环状醇与未活化的溴代烷烃的烷基化半松果醇的重排,该烷烃合并了光氧化还原和Au(I)/ Au(III)的催化作用。这种氧化还原中性重排以起两个作用的二聚Au(I)光催化剂为标志。溴代烷的光氧化还原激活和Au(III)介导的频哪醇重排与C(sp 3)–C(sp 3)的还原消除相结合;一种很少访问的反应模式。这种操作简单的方法学包含易于与[Au 2(dppm)2 ] Cl 2反应的原料在用UVA LED照射后,可提供多种酮类产品。在此转化过程中,对伯,仲和叔溴代烷烃以及一系列TMS保护的α-苯乙烯基取代的醇进行了研究。
更新日期:2017-10-25
中文翻译:

Photoredox在烷基半频哪醇重排中遇到金Lewis酸催化:具有深色面的光催化剂
已经实现了多种TMS保护的α-苯乙烯基取代的环状醇与未活化的溴代烷烃的烷基化半松果醇的重排,该烷烃合并了光氧化还原和Au(I)/ Au(III)的催化作用。这种氧化还原中性重排以起两个作用的二聚Au(I)光催化剂为标志。溴代烷的光氧化还原激活和Au(III)介导的频哪醇重排与C(sp 3)–C(sp 3)的还原消除相结合;一种很少访问的反应模式。这种操作简单的方法学包含易于与[Au 2(dppm)2 ] Cl 2反应的原料在用UVA LED照射后,可提供多种酮类产品。在此转化过程中,对伯,仲和叔溴代烷烃以及一系列TMS保护的α-苯乙烯基取代的醇进行了研究。



























 京公网安备 11010802027423号
京公网安备 11010802027423号