当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An unexpected acid-catalyzed decomposition reaction of cilnidipine and pranidipine to the decarboxylative bridged tricyclic products via cascade rearrangements
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-07-28 00:00:00 , DOI: 10.1039/c7qo00496f Minghua Yang 1, 2, 3, 4, 5 , Shusheng Zhang 4, 6, 7, 8, 9 , Xiang Zhang 4, 10, 11, 12 , Haoyang Wang 4, 6, 7, 8, 9 , Fang Zhang 4, 6, 7, 8, 9 , Yuting Hou 1, 2, 3, 4 , Yue Su 1, 2, 3, 4 , Yinlong Guo 4, 6, 7, 8, 9
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-07-28 00:00:00 , DOI: 10.1039/c7qo00496f Minghua Yang 1, 2, 3, 4, 5 , Shusheng Zhang 4, 6, 7, 8, 9 , Xiang Zhang 4, 10, 11, 12 , Haoyang Wang 4, 6, 7, 8, 9 , Fang Zhang 4, 6, 7, 8, 9 , Yuting Hou 1, 2, 3, 4 , Yue Su 1, 2, 3, 4 , Yinlong Guo 4, 6, 7, 8, 9
Affiliation
|
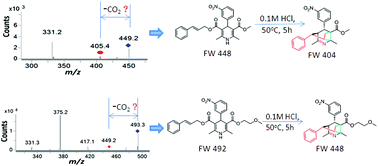
A gas-phase Carroll rearrangement occurring during electrospray ionization tandem mass spectrometry (ESI-MS/MS) led to the discovery of bridged tricyclic degradation products from cilnidipine and pranidipine under acidic conditions for the first time. The unexpected acid-catalyzed decomposition product of cilnidipine was separated and identified by MS, NMR and X-ray analyses. An acid-catalyzed Carroll rearrangement, a hetero-Diels–Alder reaction and a sigmatropic rearrangement cascade reaction were proposed for the formation of bridged tricyclic degradation products from cilnidipine and pranidipine under acidic conditions. Theoretical calculations supported the proposed reaction mechanism.
中文翻译:

西尼地平和普兰地平的意外酸催化分解反应通过级联重排成脱羧桥联的三环产物
电喷雾离子化串联质谱(ESI-MS / MS)期间发生的气相Carroll重排导致首次在酸性条件下发现了来自西尼地平和普拉尼地平的桥连三环降解产物。西尼地平的意外酸催化分解产物已分离,并通过MS,NMR和X射线分析鉴定。为了在酸性条件下由西尼地平和普拉尼地平形成桥连的三环降解产物,提出了酸催化的卡洛尔重排,杂狄尔斯-阿尔德反应和σ重排级联反应。理论计算支持了所提出的反应机理。
更新日期:2017-10-25
中文翻译:

西尼地平和普兰地平的意外酸催化分解反应通过级联重排成脱羧桥联的三环产物
电喷雾离子化串联质谱(ESI-MS / MS)期间发生的气相Carroll重排导致首次在酸性条件下发现了来自西尼地平和普拉尼地平的桥连三环降解产物。西尼地平的意外酸催化分解产物已分离,并通过MS,NMR和X射线分析鉴定。为了在酸性条件下由西尼地平和普拉尼地平形成桥连的三环降解产物,提出了酸催化的卡洛尔重排,杂狄尔斯-阿尔德反应和σ重排级联反应。理论计算支持了所提出的反应机理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号