当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
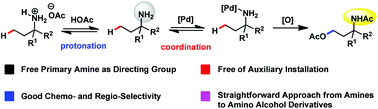
Palladium catalyzed C(sp3)–H acetoxylation of aliphatic primary amines to γ-amino alcohol derivatives
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-07-27 00:00:00 , DOI: 10.1039/c7qo00432j Kang Chen 1, 2, 3, 4, 5 , Ding Wang 1, 2, 3, 4, 5 , Zhao-Wei Li 1, 2, 3, 4, 5 , Zheng Liu 1, 2, 3, 4, 5 , Fei Pan 1, 2, 3, 4, 5 , Yun-Fei Zhang 1, 2, 3, 4, 5 , Zhang-Jie Shi 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-07-27 00:00:00 , DOI: 10.1039/c7qo00432j Kang Chen 1, 2, 3, 4, 5 , Ding Wang 1, 2, 3, 4, 5 , Zhao-Wei Li 1, 2, 3, 4, 5 , Zheng Liu 1, 2, 3, 4, 5 , Fei Pan 1, 2, 3, 4, 5 , Yun-Fei Zhang 1, 2, 3, 4, 5 , Zhang-Jie Shi 1, 2, 3, 4, 5
Affiliation
|
It still remains a major challenge to apply free primary amino groups as the directing group for aliphatic C–H functionalization. In this article, we used the protonation strategy to control the binding ability of primary amines and realized free amino group directed inert aliphatic C–H acetoxylation in good chemo- and regio-selectivity. This methodology provided a straightforward approach from primary amines to γ-amino alcohols.
中文翻译:

钯催化脂肪族伯胺的C(sp 3)–H乙酰氧基化为γ-氨基醇衍生物
应用游离的伯氨基作为脂肪族C–H功能化的导向基团仍然是一个重大挑战。在本文中,我们使用了质子化策略来控制伯胺的结合能力,并实现了具有良好的化学和区域选择性的游离氨基定向的惰性脂肪族C–H乙酰氧基化反应。该方法学提供了从伯胺到γ-氨基醇的直接方法。
更新日期:2017-10-25
中文翻译:

钯催化脂肪族伯胺的C(sp 3)–H乙酰氧基化为γ-氨基醇衍生物
应用游离的伯氨基作为脂肪族C–H功能化的导向基团仍然是一个重大挑战。在本文中,我们使用了质子化策略来控制伯胺的结合能力,并实现了具有良好的化学和区域选择性的游离氨基定向的惰性脂肪族C–H乙酰氧基化反应。该方法学提供了从伯胺到γ-氨基醇的直接方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号