当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
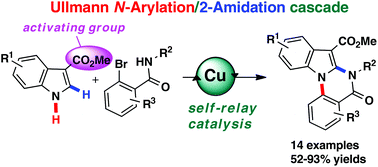
An Ullmann N-arylation/2-amidation cascade by self-relay copper catalysis: one-pot synthesis of indolo[1,2-a]quinazolinones
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-07-20 00:00:00 , DOI: 10.1039/c7qo00549k Takumi Abe 1, 2, 3, 4 , Yuka Takahashi 1, 2, 3, 4 , Yuki Matsubara 1, 2, 3, 4 , Koji Yamada 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-07-20 00:00:00 , DOI: 10.1039/c7qo00549k Takumi Abe 1, 2, 3, 4 , Yuka Takahashi 1, 2, 3, 4 , Yuki Matsubara 1, 2, 3, 4 , Koji Yamada 1, 2, 3, 4
Affiliation
|
We have developed a self-relay copper(I)-catalyzed Ullmann N-arylation/2-amidation cascade to form functionalized indolo[1,2-a]quinazolinones in one-pot from easily available indoles with 2-bromobenzamides. This novel transformation features a tandem process of Ullmann-type coupling, activation of indolenine, and 2-amidation in the presence of a single copper catalyst. In addition, among the substituents of indoles at the C3 position, methyl carboxylate could act as an activating group in this Ullmann N-arylation/2-amidation cascade.
中文翻译:

自我催化铜催化的Ullmann N-芳基化/ 2-酰胺化级联反应:一锅合成吲哚[1,2- a ]喹唑啉酮
我们已经开发了一种可自我取代的铜(I)催化的Ullmann N-芳基化/ 2-酰胺化级联反应,可从一锅中从容易获得的吲哚与2-溴苯甲酰胺一起形成官能化的吲哚[1,2- a ]喹唑啉酮。这种新颖的转化过程在单个铜催化剂的存在下具有乌尔曼型偶联,吲哚啉活化和2-酰胺化的串联过程。另外,在C3位的吲哚取代基中,羧酸甲酯可以充当该Ullmann N-芳基化/ 2-酰胺化级联反应中的活化基团。
更新日期:2017-10-25
中文翻译:

自我催化铜催化的Ullmann N-芳基化/ 2-酰胺化级联反应:一锅合成吲哚[1,2- a ]喹唑啉酮
我们已经开发了一种可自我取代的铜(I)催化的Ullmann N-芳基化/ 2-酰胺化级联反应,可从一锅中从容易获得的吲哚与2-溴苯甲酰胺一起形成官能化的吲哚[1,2- a ]喹唑啉酮。这种新颖的转化过程在单个铜催化剂的存在下具有乌尔曼型偶联,吲哚啉活化和2-酰胺化的串联过程。另外,在C3位的吲哚取代基中,羧酸甲酯可以充当该Ullmann N-芳基化/ 2-酰胺化级联反应中的活化基团。



























 京公网安备 11010802027423号
京公网安备 11010802027423号