Journal of Controlled Release ( IF 10.8 ) Pub Date : 2017-10-24 , DOI: 10.1016/j.jconrel.2017.10.032 Joanna Poutou , Maria Bunuales , Manuela Gonzalez-Aparicio , Beatriz German , Ines Zugasti , Ruben Hernandez-Alcoceba
|
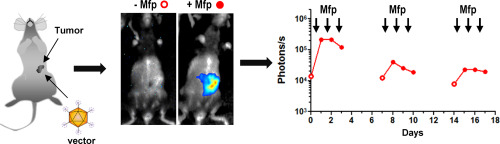
Biological therapies based on recombinant proteins such as antibodies or cytokines are continuously improving the repertoire of treatments against cancer. However, safety and efficacy of this approach is often limited by inappropriate biodistribution and pharmacokinetics of the proteins when they are administered systemically. Local administration of gene therapy vectors encoding these proteins would be a feasible alternative if they could mediate long-term and controlled expression of the transgene after a single intratumoral administration. We describe a new vector platform specially designed for this purpose. Different combinations of transactivators and promoters were evaluated to obtain a fully humanized inducible system responsive to the well-characterized drug mifepristone. The optimal transactivator conformation was based on DNA binding domains from the chimeric protein ZFHD1 fused to the progesterone receptor ligand binding domain and the NFkb p65 activation domain. The expression of this hybrid transactivator under the control of the elongation factor 1α (EF1α) or the chimeric CAG promoters ensured functionality of the system in a variety of cancer types. Expression cassettes with luciferase as a reporter gene were incorporated into High-Capacity adenoviral vectors (HC-Ad) for in vivo evaluation. Systemic administration of the vectors into C57BL/6 mice revealed that the vector based on the EF1α promoter (HCA-EF-ZP) allows tight control of transgene expression and remains stable for at least two months, whereas the CAG promoter suffers a progressive inactivation. Using an orthotopic pancreatic cancer model in syngeneic C57BL/6 mice we show that the local administration of HCA-EF-ZP achieves better tumor/liver ratio of luciferase production than the intravenous route. However, regional spread of the vector led to substantial transgene expression in peritoneal organs. We reduced this leakage through genetic modification of the vector capsid to display RGD and poly-lysine motifs in the fiber knob. Safety and antitumor effect of this gene therapy platform was demonstrated using interleukin-12 as a therapeutic gene. In conclusion, we have developed a new tool that allows local, sustained and controlled production of therapeutic proteins in tumors.
中文翻译:

适应载体和药物诱导系统以在肿瘤微环境中控制转基因的表达
基于重组蛋白(例如抗体或细胞因子)的生物疗法正在不断改善针对癌症的治疗方法。但是,这种方法的安全性和有效性通常受到全身性给药时蛋白质的不适当生物分布和药代动力学的限制。如果在单次肿瘤内给药后编码这些蛋白的基因治疗载体可以介导转基因的长期和受控表达,则将它们局部给药是可行的选择。我们描述了专门为此目的设计的新矢量平台。评价了反式激活剂和启动子的不同组合,以获得对特征明确的米非司酮有反应的完全人源化的诱导系统。最佳反式激活构象是基于嵌合蛋白ZFHD1的DNA结合结构域,该结构域与孕酮受体配体结合结构域和NFkb p65激活结构域融合。在伸长因子1α(EF1α)或嵌合CAG启动子的控制下,这种杂合反式激活子的表达确保了该系统在多种癌症类型中的功能。以荧光素酶为报告基因的表达盒被掺入高容量的腺病毒载体(HC-Ad)中。体内评估。将该载体全身性施用于C57BL / 6小鼠后发现,基于EF1α启动子的载体(HCA-EF-ZP)可以严格控制转基因的表达,并保持稳定至少两个月,而CAG启动子则逐渐失活。在同系C57BL / 6小鼠中使用原位胰腺癌模型,我们显示与静脉内途径相比,HCA-EF-ZP的局部给药可产生更好的萤光素酶肿瘤/肝脏比率。然而,载体的区域传播导致腹膜器官中大量转基因表达。我们通过对载体衣壳进行基因修饰以在纤维瘤中显示RGD和聚赖氨酸基序来减少这种泄漏。使用白介素12作为治疗基因证明了该基因治疗平台的安全性和抗肿瘤作用。综上所述,


























 京公网安备 11010802027423号
京公网安备 11010802027423号