当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Experimental Evidence for the Formation of Solvation Shells by Soluble Species at a Nonuniform Air–Ice Interface
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2017-10-23 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00077 Thorsten Bartels-Rausch 1 , Fabrizio Orlando 1 , Xiangrui Kong 1 , Luca Artiglia 1 , Markus Ammann 1
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2017-10-23 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00077 Thorsten Bartels-Rausch 1 , Fabrizio Orlando 1 , Xiangrui Kong 1 , Luca Artiglia 1 , Markus Ammann 1
Affiliation
|
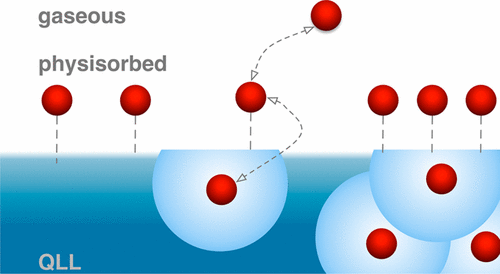
Soluble species induce surface premelting at the air–ice interface in the thermodynamic ice stability domain below the liquidus. This quasi-liquid layer is thought to increase in thickness with concentration of impurities and to represent a reservoir into which larger amounts of soluble species dissolve compared to a more rigid ice surface. To directly investigate the response of the quasi-liquid layer to increasing amounts of solutes and to clarify the distribution of these at the ice surface with depth, we use a combination of Auger electron yield X-ray absorption and photoelectron spectroscopies. We studied the adsorption of formic acid to ice at 251 K. These two complementary methods allow concluding that solutes enter the air–ice interface but do not necessarily induce a thicker quasi-liquid layer. Rather, modifications of the hydrogen-bonding network seem to be linked to the formation of solvation shells. We suggest that the flexibility of water molecules in the quasi-liquid layer is essential to form solvation shells and interpret the confinement of formic acid to the upper few ice bilayers to be linked with the structure of the hydrogen-bonding network getting more ice-like and rigid with depth at the air–ice interface.
中文翻译:

非均匀空气-冰界面上可溶性物种形成溶剂化壳的实验证据
可溶性物质在液相线以下的热力学冰稳定域中的空气-冰界面处引起表面预融化。据认为,该准液体层的厚度随杂质浓度的增加而增加,并表示与较坚硬的冰面相比,大量可溶物溶入其中的储层。为了直接研究准液层对增加的溶质的响应并阐明其在冰表面的深度分布,我们结合使用俄歇电子产X射线吸收和光电子能谱。我们研究了在251 K下甲酸在冰上的吸附。这两种互补的方法可以得出结论,溶质进入气冰界面,但不一定会引起较厚的准液层。相当,氢键网络的修饰似乎与溶剂化壳的形成有关。我们认为准液层中水分子的柔韧性对于形成溶剂化壳至关重要,并解释了甲酸在上部几个冰双层中的局限性,并与氢键网络的结构联系在一起,使冰样更像冰。在空冰界面处具有一定深度,并且具有刚性。
更新日期:2017-10-24
中文翻译:

非均匀空气-冰界面上可溶性物种形成溶剂化壳的实验证据
可溶性物质在液相线以下的热力学冰稳定域中的空气-冰界面处引起表面预融化。据认为,该准液体层的厚度随杂质浓度的增加而增加,并表示与较坚硬的冰面相比,大量可溶物溶入其中的储层。为了直接研究准液层对增加的溶质的响应并阐明其在冰表面的深度分布,我们结合使用俄歇电子产X射线吸收和光电子能谱。我们研究了在251 K下甲酸在冰上的吸附。这两种互补的方法可以得出结论,溶质进入气冰界面,但不一定会引起较厚的准液层。相当,氢键网络的修饰似乎与溶剂化壳的形成有关。我们认为准液层中水分子的柔韧性对于形成溶剂化壳至关重要,并解释了甲酸在上部几个冰双层中的局限性,并与氢键网络的结构联系在一起,使冰样更像冰。在空冰界面处具有一定深度,并且具有刚性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号