当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and Biological Evaluation of Stilbene Analogues as Hsp90 C‐Terminal Inhibitors
ChemMedChem ( IF 3.4 ) Pub Date : 2017-11-30 , DOI: 10.1002/cmdc.201700630 Katherine M Byrd 1 , Caitlin N Kent 1 , Brian S J Blagg 1
ChemMedChem ( IF 3.4 ) Pub Date : 2017-11-30 , DOI: 10.1002/cmdc.201700630 Katherine M Byrd 1 , Caitlin N Kent 1 , Brian S J Blagg 1
Affiliation
|
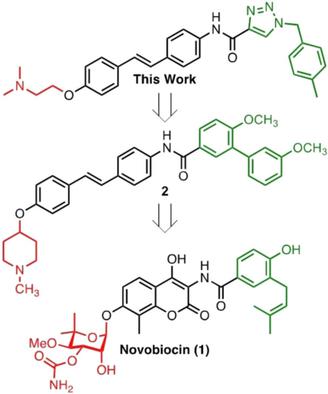
The design, synthesis, and biological evaluation of stilbene‐based novobiocin analogues is reported. Replacement of the biaryl amide side chain with a triazole side chain produced compounds that exhibited good antiproliferative activities. Heat shock protein 90 (Hsp90) inhibition was observed when N‐methylpiperidine was replaced with acyclic tertiary amines on the stilbene analogues that also contain a triazole‐derived side chain. These studies revealed that ≈24 Å is the optimal length for compounds that exhibit good antiproliferative activity as a result of Hsp90 inhibition.
中文翻译:

二苯乙烯类似物作为 Hsp90 C 末端抑制剂的合成和生物学评价
报道了基于二苯乙烯的新生霉素类似物的设计、合成和生物学评价。用三唑侧链取代联芳酰胺侧链产生的化合物表现出良好的抗增殖活性。当二苯乙烯类似物(也含有三唑衍生侧链)上的N-甲基哌啶被无环叔胺取代时,观察到热休克蛋白 90 (Hsp90) 抑制。这些研究表明,对于因 Hsp90 抑制而表现出良好抗增殖活性的化合物来说,约 24 Å 是最佳长度。
更新日期:2017-11-30
中文翻译:

二苯乙烯类似物作为 Hsp90 C 末端抑制剂的合成和生物学评价
报道了基于二苯乙烯的新生霉素类似物的设计、合成和生物学评价。用三唑侧链取代联芳酰胺侧链产生的化合物表现出良好的抗增殖活性。当二苯乙烯类似物(也含有三唑衍生侧链)上的N-甲基哌啶被无环叔胺取代时,观察到热休克蛋白 90 (Hsp90) 抑制。这些研究表明,对于因 Hsp90 抑制而表现出良好抗增殖活性的化合物来说,约 24 Å 是最佳长度。


























 京公网安备 11010802027423号
京公网安备 11010802027423号