Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A genetically inactivated two-component acellular pertussis vaccine, alone or combined with tetanus and reduced-dose diphtheria vaccines, in adolescents: a phase 2/3, randomised controlled non-inferiority trial.
The Lancet ( IF 168.9 ) Pub Date : 2018-01-01 , DOI: 10.1016/s1473-3099(17)30612-6 Sirintip Sricharoenchai , Chukiat Sirivichayakul , Kulkanya Chokephaibulkit , Punnee Pitisuttithum , Jittima Dhitavat , Arom Pitisuthitham , Wanatpreeya Phongsamart , Kobporn Boonnak , Keswadee Lapphra , Yupa Sabmee , Orasri Wittawatmongkol , Pailinrut Chinwangso , Indrajeet Kumar Poredi , Jean Petre , Pham Hong Thai , Simonetta Viviani
The Lancet ( IF 168.9 ) Pub Date : 2018-01-01 , DOI: 10.1016/s1473-3099(17)30612-6 Sirintip Sricharoenchai , Chukiat Sirivichayakul , Kulkanya Chokephaibulkit , Punnee Pitisuttithum , Jittima Dhitavat , Arom Pitisuthitham , Wanatpreeya Phongsamart , Kobporn Boonnak , Keswadee Lapphra , Yupa Sabmee , Orasri Wittawatmongkol , Pailinrut Chinwangso , Indrajeet Kumar Poredi , Jean Petre , Pham Hong Thai , Simonetta Viviani
|
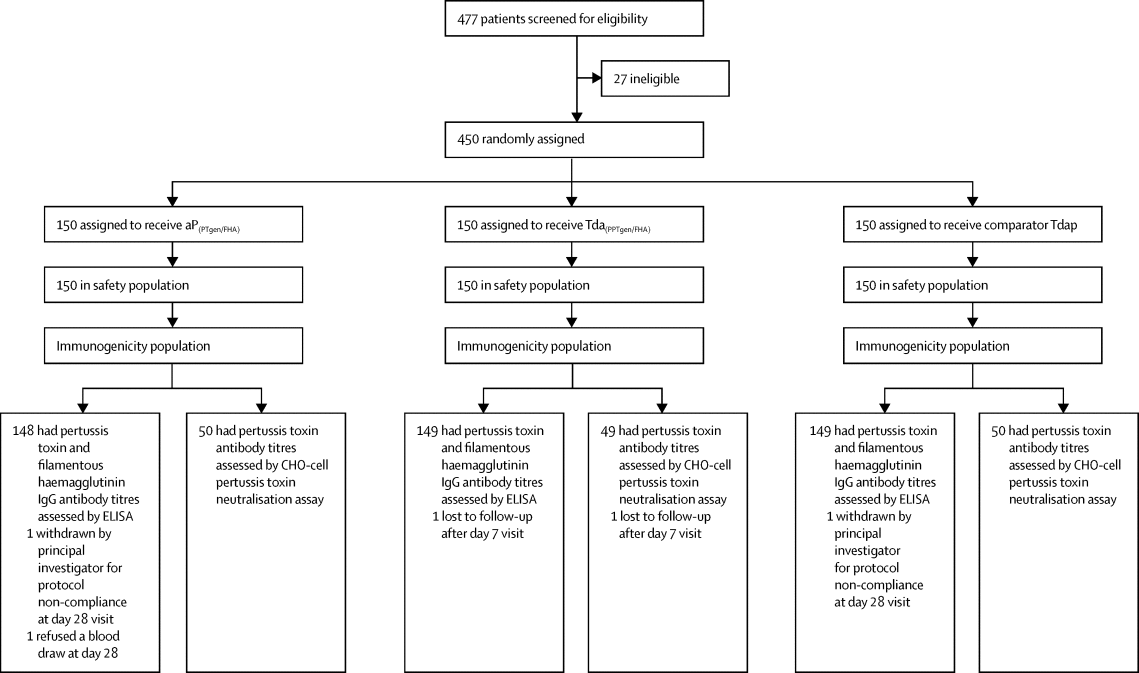
Increasing evidence shows that protection induced by acellular pertussis vaccines is short-lived, requiring repeated booster vaccination to control pertussis disease. We aimed to assess the safety and immunogenicity of a recombinant acellular pertussis vaccine containing genetically inactivated pertussis toxin and filamentous haemagglutinin, as either a monovalent vaccine (aP[PTgen/FHA]) or in combination with tetanus and reduced-dose diphtheria vaccines (TdaP[PTgen/FHA]), versus a licensed tetanus and reduced-dose diphtheria and acellular pertussis combination vaccine (Tdap).
中文翻译:

遗传灭活的两成分脱细胞百日咳疫苗,单独或与破伤风和减量白喉疫苗联合使用:2/3期随机对照非劣效性试验。
越来越多的证据表明,脱细胞百日咳疫苗诱导的保护作用是短暂的,需要反复加强疫苗接种以控制百日咳疾病。我们旨在评估包含基因灭活的百日咳毒素和丝状血凝素的重组无细胞百日咳疫苗的安全性和免疫原性,它是单价疫苗(aP [PTgen / FHA])或与破伤风和减剂量白喉疫苗(TdaP [ PTgen / FHA]),而不是许可的破伤风疫苗和减量白喉和脱细胞百日咳组合疫苗(Tdap)。
更新日期:2017-12-21
中文翻译:

遗传灭活的两成分脱细胞百日咳疫苗,单独或与破伤风和减量白喉疫苗联合使用:2/3期随机对照非劣效性试验。
越来越多的证据表明,脱细胞百日咳疫苗诱导的保护作用是短暂的,需要反复加强疫苗接种以控制百日咳疾病。我们旨在评估包含基因灭活的百日咳毒素和丝状血凝素的重组无细胞百日咳疫苗的安全性和免疫原性,它是单价疫苗(aP [PTgen / FHA])或与破伤风和减剂量白喉疫苗(TdaP [ PTgen / FHA]),而不是许可的破伤风疫苗和减量白喉和脱细胞百日咳组合疫苗(Tdap)。

























 京公网安备 11010802027423号
京公网安备 11010802027423号