当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydrogel Tethering Enhances Interdomain Stabilization of Single-Chain Antibodies
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2017-10-20 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00512 Yijia Xiong 1 , Nicole R. Ford 2 , Karen A. Hecht 2 , Guritno Roesijadi 2, 3 , Thomas C. Squier 1
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2017-10-20 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00512 Yijia Xiong 1 , Nicole R. Ford 2 , Karen A. Hecht 2 , Guritno Roesijadi 2, 3 , Thomas C. Squier 1
Affiliation
|
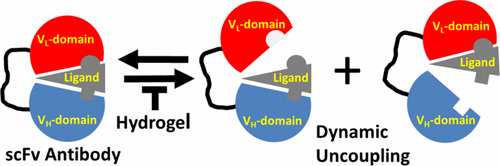
Here, we identify the importance of molecular crowding agents in the functional stabilization of scFv antibodies. Antibodies were tethered through an engineered calmodulin (CaM)-binding peptide into a stimulus-responsive hydrogel composed of poly(ethylene glycol) (PEG)-functionalized CaM. Macromolecular crowding is modulated by transient heating, which decreases effective pore sizes. Using a fluorescent ligand bound to the scFv, frequency-domain fluorescence spectroscopy was used to assess the structural coupling between the VH and the VL domains and relationships with functional stabilization. There is minimal structural coupling between the VH and the VL domains in solution, as is apparent from the substantial rotational mobility for the bound ligand, that is suggestive of an independent mobility for the VH and the VL domains. In comparison, the hydrogel matrix acts to structurally couple the VH and the VL domains, resulting in a reduction in rotational mobility and a retention of ligand binding in the presence of 8.0 M urea. Under these same conditions, ligand binding is disrupted for scFv antibodies in solution. Increases in the stabilization of scFv antibodies in hydrogels is not simply the result of molecular crowding because decreases in pore size act to destabilize ligand binding. Rather, our results suggest that the functional stabilization of the scFv antibody within the PEG hydrogel matrix includes important factors involving protein solvation that stabilize interdomain interactions between the VH and the VL domains necessary for ligand binding.
中文翻译:

水凝胶束缚增强单链抗体的域间稳定。
在这里,我们确定分子拥挤剂在scFv抗体功能稳定中的重要性。通过工程化的钙调蛋白(CaM)结合肽将抗体束缚到由聚(乙二醇)(PEG)官能化的CaM组成的刺激响应水凝胶中。大分子拥挤通过瞬时加热来调节,这减小了有效孔径。使用与scFv结合的荧光配体,频域荧光光谱法用于评估V H和V L结构域之间的结构偶联以及与功能稳定的关系。V H和V L之间的结构耦合最小从对于结合的配体的基本旋转迁移率可以明显看出,这表明溶液中的多个结构域,这暗示了对于V H和V L结构域的独立迁移率。相比之下,水凝胶基质的作用是在结构上耦合V H和V L在8.0M尿素的存在下导致旋转运动性降低和配体结合的保留。在这些相同条件下,溶液中的scFv抗体的配体结合被破坏。水凝胶中scFv抗体稳定性的提高不仅仅是分子拥挤的结果,因为孔径的减小会破坏配体结合的稳定性。相反,我们的结果表明,scFv抗体在PEG水凝胶基质中的功能稳定性包括涉及蛋白质溶剂化的重要因素,这些因素可稳定配体结合所需的V H和V L结构域之间的结构域间相互作用。
更新日期:2017-10-20
中文翻译:

水凝胶束缚增强单链抗体的域间稳定。
在这里,我们确定分子拥挤剂在scFv抗体功能稳定中的重要性。通过工程化的钙调蛋白(CaM)结合肽将抗体束缚到由聚(乙二醇)(PEG)官能化的CaM组成的刺激响应水凝胶中。大分子拥挤通过瞬时加热来调节,这减小了有效孔径。使用与scFv结合的荧光配体,频域荧光光谱法用于评估V H和V L结构域之间的结构偶联以及与功能稳定的关系。V H和V L之间的结构耦合最小从对于结合的配体的基本旋转迁移率可以明显看出,这表明溶液中的多个结构域,这暗示了对于V H和V L结构域的独立迁移率。相比之下,水凝胶基质的作用是在结构上耦合V H和V L在8.0M尿素的存在下导致旋转运动性降低和配体结合的保留。在这些相同条件下,溶液中的scFv抗体的配体结合被破坏。水凝胶中scFv抗体稳定性的提高不仅仅是分子拥挤的结果,因为孔径的减小会破坏配体结合的稳定性。相反,我们的结果表明,scFv抗体在PEG水凝胶基质中的功能稳定性包括涉及蛋白质溶剂化的重要因素,这些因素可稳定配体结合所需的V H和V L结构域之间的结构域间相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号