当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Phosphaethynolate Dimerization and Carbonyl Migration in Cyclopentadienyliron Carbonyl Systems: A Theoretical Study
Organometallics ( IF 2.8 ) Pub Date : 2017-10-19 00:00:00 , DOI: 10.1021/acs.organomet.7b00467 Chencheng Liu 1 , Yang Liu 1 , Ge Tian 1 , Liang Pu 1 , Zhong Zhang 1 , Song Yang 2 , R. Bruce King 3
Organometallics ( IF 2.8 ) Pub Date : 2017-10-19 00:00:00 , DOI: 10.1021/acs.organomet.7b00467 Chencheng Liu 1 , Yang Liu 1 , Ge Tian 1 , Liang Pu 1 , Zhong Zhang 1 , Song Yang 2 , R. Bruce King 3
Affiliation
|
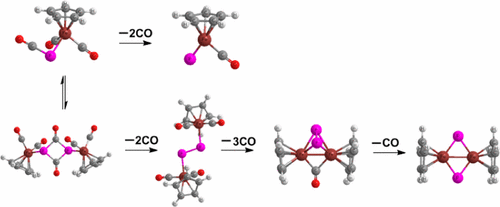
Successive decarbonylation and cyclodimerization of the mono- and binuclear FeCp(PCO)(CO)2, Fe2Cp2(PCO)2(CO)4, and Fe2Cp2(CO)2(P2) complexes have been investigated using density functional theory calculations at the M06L/DZP level. For the mononuclear complexes, the lowest energy FeCp(PCO)(CO)m (m = 2, 1) structures always prefer the η-P(CO) bent configuration relative to other models. However, the lowest energy structure for the species of stoichiometry FeCp(PCO) is actually FeCp(P)(CO) with a formal Fe≡P triple bond. The binuclear complexes are much more complicated. The Fe2Cp2(PCO)2(CO)4 structure, highly entropy controlled, has a P2C2 ring originating from cyclodimerization of two P═C═O groups from two FeCp(PCO)(CO)2 monomers. The lowest energy Fe2Cp2(PCO)2(CO)3 structure has a μ-P(CO) group donating two lone-pair electrons to each iron atom in a nonbonded Fe···Fe unit. The most favorable structure for the species of stoichiometry Fe2Cp2(PCO)2(CO)2 has an end-to-end diphosphene P2 bridge connecting two FeCp(CO) fragments in a trans arrangement. The lowest energy Fe2Cp2(PCO)2(CO) structure has μ-P(P) and μ-C(O) groups bridging an Fe–Fe single bond. The lowest energy structures for the species of stoichiometries Fe2Cp2(P2)(CO)2 and Fe2Cp2(P2)(CO) have a central P2Fe2 tetrahedron as well as two or one CO group(s) bridging Fe–Fe or Fe═Fe bonds. The lowest energy carbonyl-free Fe2Cp2P2 structure has a rhombic Fe2P2 core with no direct P–P bond.
中文翻译:

环戊二烯基铁羰基体系中的磷乙炔酸酯二聚和羰基迁移:理论研究
已经研究了单核和双核FeCp(PCO)(CO)2,Fe 2 Cp 2(PCO)2(CO)4和Fe 2 Cp 2(CO)2(P 2)配合物的连续脱羰和环二聚化作用。M06L / DZP级别的密度泛函理论计算。对于单核络合物,最低能量FeCp(PCO)(CO)m(m= 2,1)相对于其他模型,结构始终首选η-P(CO)弯曲构造。但是,化学计量FeCp(PCO)种类的最低能量结构实际上是具有正式Fe≡P三键的FeCp(P)(CO)。双核复合物要复杂得多。具有高度熵控制的Fe 2 Cp 2(PCO)2(CO)4结构具有一个P 2 C 2环,该环来自两个FeCp(PCO)(CO)2单体的两个P═C═O基团的环二聚。最低能量Fe 2 Cp 2(PCO)2(CO)3该结构具有一个μ-P(CO)基团,该基团在未键合的Fe···Fe单元中为每个铁原子提供两个孤对电子。对于化学计量的物种Fe 2 Cp 2(PCO)2(CO)2而言,最有利的结构具有以反式连接方式连接两个FeCp(CO)片段的首尾相连的二膦P 2桥。最低能量的Fe 2 Cp 2(PCO)2(CO)结构具有连接Fe–Fe单键的μ-P(P)和μ-C(O)基团。化学计量比为Fe 2 Cp 2(P 2)(CO)2和Fe 2 Cp 2的物种的最低能级结构(P 2)(CO)具有一个中心P 2 Fe 2四面体以及两个或一个桥接Fe–Fe或Fe═Fe键的CO基团。能量最低的无羰基Fe 2 Cp 2 P 2结构具有菱形的Fe 2 P 2核,没有直接的P–P键。
更新日期:2017-10-20
中文翻译:

环戊二烯基铁羰基体系中的磷乙炔酸酯二聚和羰基迁移:理论研究
已经研究了单核和双核FeCp(PCO)(CO)2,Fe 2 Cp 2(PCO)2(CO)4和Fe 2 Cp 2(CO)2(P 2)配合物的连续脱羰和环二聚化作用。M06L / DZP级别的密度泛函理论计算。对于单核络合物,最低能量FeCp(PCO)(CO)m(m= 2,1)相对于其他模型,结构始终首选η-P(CO)弯曲构造。但是,化学计量FeCp(PCO)种类的最低能量结构实际上是具有正式Fe≡P三键的FeCp(P)(CO)。双核复合物要复杂得多。具有高度熵控制的Fe 2 Cp 2(PCO)2(CO)4结构具有一个P 2 C 2环,该环来自两个FeCp(PCO)(CO)2单体的两个P═C═O基团的环二聚。最低能量Fe 2 Cp 2(PCO)2(CO)3该结构具有一个μ-P(CO)基团,该基团在未键合的Fe···Fe单元中为每个铁原子提供两个孤对电子。对于化学计量的物种Fe 2 Cp 2(PCO)2(CO)2而言,最有利的结构具有以反式连接方式连接两个FeCp(CO)片段的首尾相连的二膦P 2桥。最低能量的Fe 2 Cp 2(PCO)2(CO)结构具有连接Fe–Fe单键的μ-P(P)和μ-C(O)基团。化学计量比为Fe 2 Cp 2(P 2)(CO)2和Fe 2 Cp 2的物种的最低能级结构(P 2)(CO)具有一个中心P 2 Fe 2四面体以及两个或一个桥接Fe–Fe或Fe═Fe键的CO基团。能量最低的无羰基Fe 2 Cp 2 P 2结构具有菱形的Fe 2 P 2核,没有直接的P–P键。



























 京公网安备 11010802027423号
京公网安备 11010802027423号