PLoS Pathogens ( IF 6.7 ) Pub Date : 2017-10-19 , DOI: 10.1371/journal.ppat.1006571 Orin Courtenay 1, 2 , Nathan C Peters 3 , Matthew E Rogers 4 , Caryn Bern 5
|
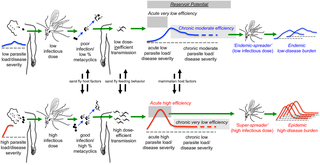
Quantitation of the nonlinear heterogeneities in Leishmania parasites, sand fly vectors, and mammalian host relationships provides insights to better understand leishmanial transmission epidemiology towards improving its control. The parasite manipulates the sand fly via production of promastigote secretory gel (PSG), leading to the “blocked sand fly” phenotype, persistent feeding attempts, and feeding on multiple hosts. PSG is injected into the mammalian host with the parasite and promotes the establishment of infection. Animal models demonstrate that sand flies with the highest parasite loads and percent metacyclic promastigotes transmit more parasites with greater frequency, resulting in higher load infections that are more likely to be both symptomatic and efficient reservoirs. The existence of mammalian and sand fly “super-spreaders” provides a biological basis for the spatial and temporal clustering of clinical leishmanial disease. Sand fly blood-feeding behavior will determine the efficacies of indoor residual spraying, topical insecticides, and bed nets. Interventions need to have sufficient coverage to include transmission hot spots, especially in the absence of field tools to assess infectiousness. Interventions that reduce sand fly densities in the absence of elimination could have negative consequences, for example, by interfering with partial immunity conferred by exposure to sand fly saliva. A deeper understanding of both sand fly and host biology and behavior is essential to ensuring effectiveness of vector interventions.
中文翻译:

将流行病学与白蛉、寄生虫和宿主的基础生物学相结合,为利什曼病传播动力学和控制提供信息
利什曼原虫非线性异质性的定量寄生虫、沙蝇媒介和哺乳动物宿主关系为更好地了解利什曼原虫传播流行病学以改善其控制提供了见解。寄生虫通过产生前鞭毛体分泌凝胶 (PSG) 来操纵沙蝇,导致“阻塞沙蝇”表型、持续的摄食尝试和以多个宿主为食。PSG 与寄生虫一起注射到哺乳动物宿主中并促进感染的建立。动物模型表明,具有最高寄生虫负荷和亚环前鞭毛体百分比的沙蝇以更高的频率传播更多的寄生虫,从而导致更高负荷的感染,这些感染更有可能是有症状的和有效的宿主。哺乳动物和沙蝇“超级传播者”的存在为临床利什曼病的时空聚集提供了生物学基础。沙蝇吸血行为将决定室内滞留喷洒、外用杀虫剂和蚊帐的效果。干预措施需要有足够的覆盖范围以包括传播热点,尤其是在缺乏现场工具来评估传染性的情况下。在没有消除的情况下降低沙蝇密度的干预措施可能会产生负面影响,例如,通过干扰暴露于沙蝇唾液所赋予的部分免疫力。更深入地了解沙蝇和宿主生物学和行为对于确保媒介干预的有效性至关重要。沙蝇吸血行为将决定室内滞留喷洒、外用杀虫剂和蚊帐的效果。干预措施需要有足够的覆盖范围以包括传播热点,尤其是在缺乏现场工具来评估传染性的情况下。在没有消除的情况下降低沙蝇密度的干预措施可能会产生负面影响,例如,通过干扰暴露于沙蝇唾液所赋予的部分免疫力。更深入地了解沙蝇和宿主生物学和行为对于确保媒介干预的有效性至关重要。沙蝇吸血行为将决定室内滞留喷洒、外用杀虫剂和蚊帐的效果。干预措施需要有足够的覆盖范围以包括传播热点,尤其是在缺乏现场工具来评估传染性的情况下。在没有消除的情况下降低沙蝇密度的干预措施可能会产生负面影响,例如,通过干扰暴露于沙蝇唾液所赋予的部分免疫力。更深入地了解沙蝇和宿主生物学和行为对于确保媒介干预的有效性至关重要。在没有消除的情况下降低沙蝇密度的干预措施可能会产生负面影响,例如,通过干扰暴露于沙蝇唾液所赋予的部分免疫力。更深入地了解沙蝇和宿主生物学和行为对于确保媒介干预的有效性至关重要。在没有消除的情况下降低沙蝇密度的干预措施可能会产生负面影响,例如,通过干扰暴露于沙蝇唾液所赋予的部分免疫力。更深入地了解沙蝇和宿主生物学和行为对于确保媒介干预的有效性至关重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号