当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Highly active, stable oxidized platinum clusters as electrocatalysts for the hydrogen evolution reaction
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2017-10-11 00:00:00 , DOI: 10.1039/c7ee02537h Xing Cheng 1, 2, 3, 4, 5 , Yonghe Li 3, 5, 6, 7 , Lirong Zheng 5, 8, 9, 10, 11 , Yong Yan 5, 12, 13, 14 , Yuefei Zhang 3, 5, 6, 7 , Ge Chen 1, 2, 3, 4, 5 , Shaorui Sun 1, 2, 3, 4, 5 , Jiujun Zhang 5, 15, 16, 17
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2017-10-11 00:00:00 , DOI: 10.1039/c7ee02537h Xing Cheng 1, 2, 3, 4, 5 , Yonghe Li 3, 5, 6, 7 , Lirong Zheng 5, 8, 9, 10, 11 , Yong Yan 5, 12, 13, 14 , Yuefei Zhang 3, 5, 6, 7 , Ge Chen 1, 2, 3, 4, 5 , Shaorui Sun 1, 2, 3, 4, 5 , Jiujun Zhang 5, 15, 16, 17
Affiliation
|
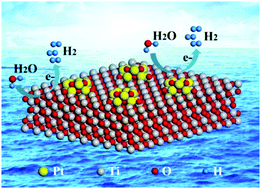
In the present work, we synthesized and characterized an electrocatalyst consisting of sub-nanometric Pt clusters uniformly dispersed on a TiO2 support. X-ray photoelectron spectra (XPS) and X-ray adsorption fine structure (XAFS) data demonstrate that these sub-nanometric Pt clusters are in a highly oxidized state and possess two localized Pt–O coordination structures. The Pt–O bonds between the oxidized Pt clusters and the TiO2 give rise to a strong metal–support interaction (SMSI). When applied to the hydrogen evolution reaction (HER), this catalyst exhibits significantly enhanced catalytic activity (increased by a factor of up to 8.4) and enhanced stability compared with the state-of-the-art commercial Pt/C catalysts. Particularly, the additional XPS and XAFS characterizations of the catalyst after long-term electrolysis demonstrate the absence of metallic Pt species, confirming that the catalytic active site comes from the oxidized Pt clusters rather than from the the metallic Pt species. This improved performance is considered to be induced by the unique electronic structure of the oxidized Pt clusters and by the SMSI. Based on the results of density functional theory calculations, the 5d orbital of the oxidized Pt cluster atoms appears to hybridize with the H 1s orbital to form weak Pt–H valence bonds, leading to a ΔG (relative free energy) value of approximately zero eV for H* absorption. This effect explains the mechanism responsible for the excellent catalytic activity of these oxidized Pt clusters for the HER. This work therefore provides important insights into the role of oxidized Pt clusters as an HER electrocatalyst. The evident stabilization of the oxidized Pt clusters on TiO2 supports via the charge-transfer mechanism provides a useful approach for improving the durability of electrocatalysts that may be applicable to other noble metal/support systems.
中文翻译:

高活性,稳定的氧化铂簇作为氢析出反应的电催化剂
在本工作中,我们合成并表征了由亚纳米Pt团簇均匀分散在TiO 2载体上组成的电催化剂。X射线光电子能谱(XPS)和X射线吸附精细结构(XAFS)数据表明,这些亚纳米级的Pt团簇处于高度氧化态,并具有两个局部的Pt-O配位结构。氧化的Pt团簇与TiO 2之间的Pt–O键产生强大的金属-支撑相互作用(SMSI)。与最先进的市售Pt / C催化剂相比,将该催化剂应用于放氢反应(HER)时,具有显着增强的催化活性(提高了8.4倍)和增强的稳定性。特别地,长期电解后催化剂的其他XPS和XAFS表征表明不存在金属Pt物种,这证实了催化活性位点来自氧化的Pt簇而不是金属Pt物种。认为这种改善的性能是由氧化的Pt团簇的独特电子结构和SMSI引起的。根据密度泛函理论计算的结果,H *吸收的G(相对自由能)值约为零eV。这种效应解释了这些氧化的Pt簇对HER具有出色催化活性的机理。因此,这项工作为氧化Pt簇作为HER电催化剂的作用提供了重要的见识。经由电荷转移机理,TiO 2载体上氧化的Pt团簇的明显稳定化为提高可用于其他贵金属/载体系统的电催化剂的耐久性提供了一种有用的方法。
更新日期:2017-10-19
中文翻译:

高活性,稳定的氧化铂簇作为氢析出反应的电催化剂
在本工作中,我们合成并表征了由亚纳米Pt团簇均匀分散在TiO 2载体上组成的电催化剂。X射线光电子能谱(XPS)和X射线吸附精细结构(XAFS)数据表明,这些亚纳米级的Pt团簇处于高度氧化态,并具有两个局部的Pt-O配位结构。氧化的Pt团簇与TiO 2之间的Pt–O键产生强大的金属-支撑相互作用(SMSI)。与最先进的市售Pt / C催化剂相比,将该催化剂应用于放氢反应(HER)时,具有显着增强的催化活性(提高了8.4倍)和增强的稳定性。特别地,长期电解后催化剂的其他XPS和XAFS表征表明不存在金属Pt物种,这证实了催化活性位点来自氧化的Pt簇而不是金属Pt物种。认为这种改善的性能是由氧化的Pt团簇的独特电子结构和SMSI引起的。根据密度泛函理论计算的结果,H *吸收的G(相对自由能)值约为零eV。这种效应解释了这些氧化的Pt簇对HER具有出色催化活性的机理。因此,这项工作为氧化Pt簇作为HER电催化剂的作用提供了重要的见识。经由电荷转移机理,TiO 2载体上氧化的Pt团簇的明显稳定化为提高可用于其他贵金属/载体系统的电催化剂的耐久性提供了一种有用的方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号