当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
USP7-Specific Inhibitors Target and Modify the Enzyme's Active Site via Distinct Chemical Mechanisms
Cell Chemical Biology ( IF 8.6 ) Pub Date : 2017-10-19 , DOI: 10.1016/j.chembiol.2017.09.004 Alexandra Pozhidaeva , Gabrielle Valles , Feng Wang , Jian Wu , David E. Sterner , Phuong Nguyen , Joseph Weinstock , K.G. Suresh Kumar , Jean Kanyo , Dennis Wright , Irina Bezsonova
Cell Chemical Biology ( IF 8.6 ) Pub Date : 2017-10-19 , DOI: 10.1016/j.chembiol.2017.09.004 Alexandra Pozhidaeva , Gabrielle Valles , Feng Wang , Jian Wu , David E. Sterner , Phuong Nguyen , Joseph Weinstock , K.G. Suresh Kumar , Jean Kanyo , Dennis Wright , Irina Bezsonova
|
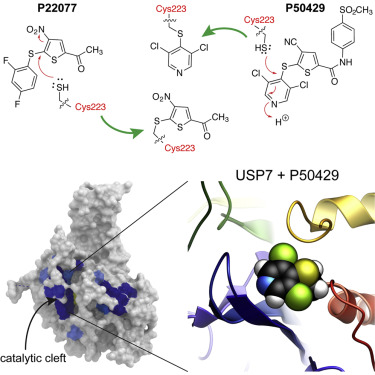
USP7 is a deubiquitinating enzyme that plays a pivotal role in multiple oncogenic pathways and therefore is a desirable target for new anti-cancer therapies. However, the lack of structural information about the USP7-inhibitor interactions has been a critical gap in the development of potent inhibitors. USP7 is unique among USPs in that its active site is catalytically incompetent, and is postulated to rearrange into a productive conformation only upon binding to ubiquitin. Surprisingly, we found that ubiquitin alone does not induce an active conformation in solution. Using a combination of nuclear magnetic resonance, mass spectrometry, computational modeling, and cell-based assays, we found that DUB inhibitors P22077 and P50429 covalently modify the catalytic cysteine of USP7 and induce a conformational switch in the enzyme associated with active site rearrangement. This work represents the first experimental insights into USP7 activation and inhibition and provides a structural basis for rational development of potent anti-cancer therapeutics.
中文翻译:

USP7特异的抑制剂通过不同的化学机制靶向和修饰酶的活性位点
USP7是一种去泛素化酶,在多种致癌途径中起关键作用,因此是新的抗癌治疗的理想靶标。但是,缺乏有关USP7-抑制剂相互作用的结构信息一直是开发有效抑制剂的关键缺口。USP7在USP中是独特的,因为它的活性位点在催化上是不能胜任的,并且假定仅在与泛素结合后才能重新排列成有效的构象。令人惊讶地,我们发现单独的遍在蛋白不会在溶液中诱导活性构象。结合使用核磁共振,质谱,计算模型和基于细胞的分析,我们发现DUB抑制剂P22077和P50429共价修饰USP7的催化半胱氨酸并诱导与活性位点重排相关的酶的构象转换。这项工作代表了对USP7激活和抑制的第一个实验性见识,并为合理开发有效的抗癌疗法提供了结构基础。
更新日期:2017-12-21
中文翻译:

USP7特异的抑制剂通过不同的化学机制靶向和修饰酶的活性位点
USP7是一种去泛素化酶,在多种致癌途径中起关键作用,因此是新的抗癌治疗的理想靶标。但是,缺乏有关USP7-抑制剂相互作用的结构信息一直是开发有效抑制剂的关键缺口。USP7在USP中是独特的,因为它的活性位点在催化上是不能胜任的,并且假定仅在与泛素结合后才能重新排列成有效的构象。令人惊讶地,我们发现单独的遍在蛋白不会在溶液中诱导活性构象。结合使用核磁共振,质谱,计算模型和基于细胞的分析,我们发现DUB抑制剂P22077和P50429共价修饰USP7的催化半胱氨酸并诱导与活性位点重排相关的酶的构象转换。这项工作代表了对USP7激活和抑制的第一个实验性见识,并为合理开发有效的抗癌疗法提供了结构基础。


























 京公网安备 11010802027423号
京公网安备 11010802027423号