当前位置:
X-MOL 学术
›
Cancer Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
mTORC1 Couples Nucleotide Synthesis to Nucleotide Demand Resulting in a Targetable Metabolic Vulnerability.
Cancer Cell ( IF 50.3 ) Pub Date : 2017-11-13 , DOI: 10.1016/j.ccell.2017.09.013 Alexander J. Valvezan , Marc Turner , Amine Belaid , Hilaire C. Lam , Spencer K. Miller , Molly C. McNamara , Christian Baglini , Benjamin E. Housden , Norbert Perrimon , David J. Kwiatkowski , John M. Asara , Elizabeth P. Henske , Brendan D. Manning
Cancer Cell ( IF 50.3 ) Pub Date : 2017-11-13 , DOI: 10.1016/j.ccell.2017.09.013 Alexander J. Valvezan , Marc Turner , Amine Belaid , Hilaire C. Lam , Spencer K. Miller , Molly C. McNamara , Christian Baglini , Benjamin E. Housden , Norbert Perrimon , David J. Kwiatkowski , John M. Asara , Elizabeth P. Henske , Brendan D. Manning
|
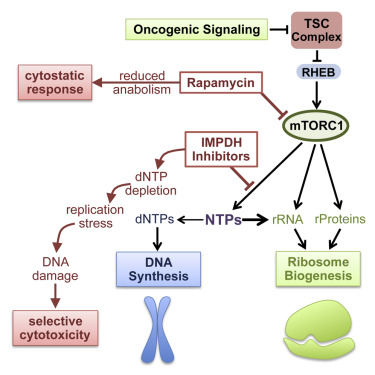
The mechanistic target of rapamycin complex 1 (mTORC1) supports proliferation through parallel induction of key anabolic processes, including protein, lipid, and nucleotide synthesis. We hypothesized that these processes are coupled to maintain anabolic balance in cells with mTORC1 activation, a common event in human cancers. Loss of the tuberous sclerosis complex (TSC) tumor suppressors results in activation of mTORC1 and development of the tumor syndrome TSC. We find that pharmacological inhibitors of guanylate nucleotide synthesis have selective deleterious effects on TSC-deficient cells, including in mouse tumor models. This effect stems from replication stress and DNA damage caused by mTORC1-driven rRNA synthesis, which renders nucleotide pools limiting. These findings reveal a metabolic vulnerability downstream of mTORC1 triggered by anabolic imbalance.
中文翻译:

mTORC1将核苷酸合成与核苷酸需求耦合,从而导致目标代谢脆弱性。
雷帕霉素复合物1(mTORC1)的机械靶标通过关键合成代谢过程的并行诱导(包括蛋白质,脂质和核苷酸合成)支持增殖。我们假设这些过程耦合在一起以维持mTORC1活化的细胞中合成代谢的平衡,而mTORC1活化是人类癌症中的常见事件。结节性硬化复合物(TSC)肿瘤抑制因子的丧失导致mTORC1活化和肿瘤综合征TSC的发展。我们发现鸟苷酸核苷酸合成的药理抑制剂对TSC缺陷型细胞具有选择性有害作用,包括在小鼠肿瘤模型中。这种效应源于由mTORC1驱动的rRNA合成引起的复制压力和DNA损伤,这使核苷酸池受到限制。
更新日期:2017-10-19
中文翻译:

mTORC1将核苷酸合成与核苷酸需求耦合,从而导致目标代谢脆弱性。
雷帕霉素复合物1(mTORC1)的机械靶标通过关键合成代谢过程的并行诱导(包括蛋白质,脂质和核苷酸合成)支持增殖。我们假设这些过程耦合在一起以维持mTORC1活化的细胞中合成代谢的平衡,而mTORC1活化是人类癌症中的常见事件。结节性硬化复合物(TSC)肿瘤抑制因子的丧失导致mTORC1活化和肿瘤综合征TSC的发展。我们发现鸟苷酸核苷酸合成的药理抑制剂对TSC缺陷型细胞具有选择性有害作用,包括在小鼠肿瘤模型中。这种效应源于由mTORC1驱动的rRNA合成引起的复制压力和DNA损伤,这使核苷酸池受到限制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号