当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Heparin Binding to an Engineered Virus-like Nanoparticle Antagonist
Biomacromolecules ( IF 6.2 ) Pub Date : 2017-10-18 00:00:00 , DOI: 10.1021/acs.biomac.7b01174 Ho Yong Cheong 1 , Myles Groner 1 , Kevin Hong 1 , Brennen Lynch 1 , William R. Hollingsworth 1 , Zinaida Polonskaya 2 , Jin-Kyu Rhee 3 , Michael M. Baksh 4 , M. G. Finn 4 , Andrew J. Gale 5 , Andrew K. Udit 1
Biomacromolecules ( IF 6.2 ) Pub Date : 2017-10-18 00:00:00 , DOI: 10.1021/acs.biomac.7b01174 Ho Yong Cheong 1 , Myles Groner 1 , Kevin Hong 1 , Brennen Lynch 1 , William R. Hollingsworth 1 , Zinaida Polonskaya 2 , Jin-Kyu Rhee 3 , Michael M. Baksh 4 , M. G. Finn 4 , Andrew J. Gale 5 , Andrew K. Udit 1
Affiliation
|
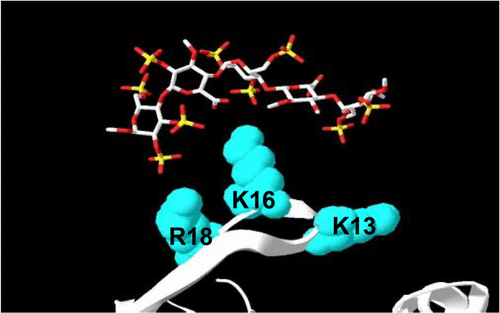
The anticoagulant activity of heparin administered during medical interventions must be reversed to restore normal clotting, typically by titrating with protamine. Given the acute toxicity associated with protamine, we endeavored to generate safer heparin antagonists by engineering bacteriophage Qβ virus-like particles (VLPs) to display motifs that bind heparin. A particle bearing a single amino acid change from wild-type (T18R) was identified as a promising candidate for heparin antagonism. Surface potential maps generated through molecular modeling reveal that the T18R mutation adds synergistically to adjacent positive charges on the particle surface, resulting in a large solvent-accessible cationic region that is replicated 180 times over the capsid. Chromatography using a heparin-sepharose column confirmed a strong interaction between heparin and the T18R particle. Binding studies using fluorescein-labeled heparin (HepFL) resulted in a concentration-dependent change in fluorescence intensity, which could be perturbed by the addition of unlabeled heparin. Analysis of the fluorescence data yielded a dissociation constant of approximately 1 nM and a 1:1 binding stoichiometry for HepFL:VLP. Dynamic light scattering (DLS) experiments suggested that T18R forms discrete complexes with heparin when the VLP:heparin molar ratios are equivalent, and in vitro clotting assays confirmed the 1:1 binding stoichiometry as full antagonism of heparin is achieved. Biolayer interferometry and backscattering interferometry corroborated the strong interaction of T18R with heparin, yielding Kd ∼ 1–10 nM. These biophysical measurements further validated T18R, and VLPs in general, for potential clinical use as effective, nontoxic heparin antagonists.
中文翻译:

肝素结合工程病毒样纳米粒子拮抗剂。
通常必须通过用鱼精蛋白滴定来逆转医学干预期间给予的肝素的抗凝活性,以恢复正常的凝血。考虑到与鱼精蛋白相关的急性毒性,我们努力通过工程化噬菌体Qβ病毒样颗粒(VLP)来显示结合肝素的基序,从而产生更安全的肝素拮抗剂。从野生型(T18R)携带单个氨基酸改变的颗粒被确定为肝素拮抗作用的有希望的候选者。通过分子建模生成的表面电势图显示,T18R突变可协同添加到颗粒表面上的相邻正电荷上,从而产生一个大的溶剂可及的阳离子区域,该区域在衣壳上复制了180次。使用肝素-琼脂糖柱的色谱法确证了肝素和T18R颗粒之间的强相互作用。使用荧光素标记的肝素(HepFL)的结合研究导致荧光强度的浓度依赖性变化,这可能会因添加未标记的肝素而受到干扰。荧光数据的分析产生了大约1 nM的解离常数和HepFL:VLP的1:1结合化学计量。动态光散射(DLS)实验表明,当VLP:肝素摩尔比相等时,T18R与肝素形成离散的复合物,并且体外凝结测定法证实了1:1的化学计量比可以实现肝素的完全拮抗作用。生物层干涉法和反向散射干涉法证实了T18R与肝素的强相互作用,产生了 使用荧光素标记的肝素(HepFL)的结合研究导致荧光强度的浓度依赖性变化,这可能会因添加未标记的肝素而受到干扰。荧光数据的分析产生了大约1 nM的解离常数和HepFL:VLP的1:1结合化学计量。动态光散射(DLS)实验表明,当VLP:肝素摩尔比相等时,T18R与肝素形成离散的复合物,并且体外凝结测定法证实了1:1的化学计量比可以实现肝素的完全拮抗作用。生物层干涉法和反向散射干涉法证实了T18R与肝素的强相互作用,产生了 使用荧光素标记的肝素(HepFL)的结合研究导致荧光强度的浓度依赖性变化,这可能会因添加未标记的肝素而受到干扰。荧光数据的分析产生了大约1 nM的解离常数和HepFL:VLP的1:1结合化学计量。动态光散射(DLS)实验表明,当VLP:肝素摩尔比相等时,T18R与肝素形成离散的复合物,并且体外凝结测定法证实了1:1的化学计量比可以实现肝素的完全拮抗作用。生物层干涉法和反向散射干涉法证实了T18R与肝素的强相互作用,产生了 可能会因添加未标记的肝素而受到干扰。荧光数据的分析产生了大约1 nM的解离常数和HepFL:VLP的1:1结合化学计量。动态光散射(DLS)实验表明,当VLP:肝素摩尔比相等时,T18R与肝素形成离散的复合物,并且体外凝结测定法证实了1:1的化学计量比可以实现肝素的完全拮抗作用。生物层干涉法和反向散射干涉法证实了T18R与肝素的强相互作用,产生了 可能会因添加未标记的肝素而受到干扰。荧光数据的分析产生了大约1 nM的解离常数和HepFL:VLP的1:1结合化学计量。动态光散射(DLS)实验表明,当VLP:肝素摩尔比相等时,T18R与肝素形成离散的复合物,并且体外凝结测定法证实了1:1的化学计量比可以实现肝素的完全拮抗作用。生物层干涉法和反向散射干涉法证实了T18R与肝素的强相互作用,产生了 动态光散射(DLS)实验表明,当VLP:肝素摩尔比相等时,T18R与肝素形成离散的复合物,并且体外凝结测定法证实了1:1的化学计量比可以实现肝素的完全拮抗作用。生物层干涉法和反向散射干涉法证实了T18R与肝素的强相互作用,产生了 动态光散射(DLS)实验表明,当VLP:肝素摩尔比相等时,T18R与肝素形成离散的复合物,并且体外凝结测定法证实了1:1的化学计量比可以实现肝素的完全拮抗作用。生物层干涉法和反向散射干涉法证实了T18R与肝素的强相互作用,产生了ķ d〜1-10纳米。这些生物物理测量结果进一步验证了T18R和VLP的总体有效性,可作为有效,无毒的肝素拮抗剂用于临床。
更新日期:2017-10-19
中文翻译:

肝素结合工程病毒样纳米粒子拮抗剂。
通常必须通过用鱼精蛋白滴定来逆转医学干预期间给予的肝素的抗凝活性,以恢复正常的凝血。考虑到与鱼精蛋白相关的急性毒性,我们努力通过工程化噬菌体Qβ病毒样颗粒(VLP)来显示结合肝素的基序,从而产生更安全的肝素拮抗剂。从野生型(T18R)携带单个氨基酸改变的颗粒被确定为肝素拮抗作用的有希望的候选者。通过分子建模生成的表面电势图显示,T18R突变可协同添加到颗粒表面上的相邻正电荷上,从而产生一个大的溶剂可及的阳离子区域,该区域在衣壳上复制了180次。使用肝素-琼脂糖柱的色谱法确证了肝素和T18R颗粒之间的强相互作用。使用荧光素标记的肝素(HepFL)的结合研究导致荧光强度的浓度依赖性变化,这可能会因添加未标记的肝素而受到干扰。荧光数据的分析产生了大约1 nM的解离常数和HepFL:VLP的1:1结合化学计量。动态光散射(DLS)实验表明,当VLP:肝素摩尔比相等时,T18R与肝素形成离散的复合物,并且体外凝结测定法证实了1:1的化学计量比可以实现肝素的完全拮抗作用。生物层干涉法和反向散射干涉法证实了T18R与肝素的强相互作用,产生了 使用荧光素标记的肝素(HepFL)的结合研究导致荧光强度的浓度依赖性变化,这可能会因添加未标记的肝素而受到干扰。荧光数据的分析产生了大约1 nM的解离常数和HepFL:VLP的1:1结合化学计量。动态光散射(DLS)实验表明,当VLP:肝素摩尔比相等时,T18R与肝素形成离散的复合物,并且体外凝结测定法证实了1:1的化学计量比可以实现肝素的完全拮抗作用。生物层干涉法和反向散射干涉法证实了T18R与肝素的强相互作用,产生了 使用荧光素标记的肝素(HepFL)的结合研究导致荧光强度的浓度依赖性变化,这可能会因添加未标记的肝素而受到干扰。荧光数据的分析产生了大约1 nM的解离常数和HepFL:VLP的1:1结合化学计量。动态光散射(DLS)实验表明,当VLP:肝素摩尔比相等时,T18R与肝素形成离散的复合物,并且体外凝结测定法证实了1:1的化学计量比可以实现肝素的完全拮抗作用。生物层干涉法和反向散射干涉法证实了T18R与肝素的强相互作用,产生了 可能会因添加未标记的肝素而受到干扰。荧光数据的分析产生了大约1 nM的解离常数和HepFL:VLP的1:1结合化学计量。动态光散射(DLS)实验表明,当VLP:肝素摩尔比相等时,T18R与肝素形成离散的复合物,并且体外凝结测定法证实了1:1的化学计量比可以实现肝素的完全拮抗作用。生物层干涉法和反向散射干涉法证实了T18R与肝素的强相互作用,产生了 可能会因添加未标记的肝素而受到干扰。荧光数据的分析产生了大约1 nM的解离常数和HepFL:VLP的1:1结合化学计量。动态光散射(DLS)实验表明,当VLP:肝素摩尔比相等时,T18R与肝素形成离散的复合物,并且体外凝结测定法证实了1:1的化学计量比可以实现肝素的完全拮抗作用。生物层干涉法和反向散射干涉法证实了T18R与肝素的强相互作用,产生了 动态光散射(DLS)实验表明,当VLP:肝素摩尔比相等时,T18R与肝素形成离散的复合物,并且体外凝结测定法证实了1:1的化学计量比可以实现肝素的完全拮抗作用。生物层干涉法和反向散射干涉法证实了T18R与肝素的强相互作用,产生了 动态光散射(DLS)实验表明,当VLP:肝素摩尔比相等时,T18R与肝素形成离散的复合物,并且体外凝结测定法证实了1:1的化学计量比可以实现肝素的完全拮抗作用。生物层干涉法和反向散射干涉法证实了T18R与肝素的强相互作用,产生了ķ d〜1-10纳米。这些生物物理测量结果进一步验证了T18R和VLP的总体有效性,可作为有效,无毒的肝素拮抗剂用于临床。



























 京公网安备 11010802027423号
京公网安备 11010802027423号