Journal of Controlled Release ( IF 10.8 ) Pub Date : 2017-10-18 , DOI: 10.1016/j.jconrel.2017.10.020 Jin Wang , Bertrand Z. Yeung , Minjian Cui , Cody J. Peer , Ze Lu , William D. Figg , M. Guillaume Wientjes , Sukyung Woo , Jessie L.-S. Au
|
Purpose
Exosomes are small membrane vesicles (30–100 nm in diameter) secreted by cells into extracellular space. The present study evaluated the effect of chemotherapeutic agents on exosome production and/or release, and quantified the contribution of exosomes to intercellular drug transfer and pharmacodynamics.
Methods
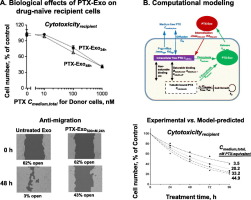
Human cancer cells (breast MCF7, breast-to-lung metastatic LM2, ovarian A2780 and OVCAR4) were treated with paclitaxel (PTX, 2–1000 nM) or doxorubicin (DOX, 20–1000 nM) for 24–48 h. Exosomes were isolated from the culture medium of drug-treated donor cells (Donor cells) using ultra-centrifugation, and analyzed for acetylcholinesterase activity, total proteins, drug concentrations, and biological effects (cytotoxicity and anti-migration) on drug-naïve recipient cells (Recipient cells). These results were used to develop computational predictive quantitative pharmacology models.
Results
Cells in exponential growth phase released ~ 220 exosomes/cell in culture medium. PTX and DOX significantly promoted exosome production and/or release in a dose- and time-dependent manner, with greater effects in ovarian cancer cells than in breast cancer cells. Exosomes isolated from Donor cells contained appreciable drug levels (2–7 pmole/106 cells after 24 h treatment with 100–1000 nM PTX), and caused cytotoxicity and inhibited migration of Recipient cells. Quantitative pharmacology models that integrated cellular PTX pharmacokinetics with PTX pharmacodynamics successfully predicted effects of exosomes on intercellular drug transfer, cytotoxicity of PTX on Donor cells and cytotoxicity of PTX-containing exosomes on Recipient cells. Additional model simulations indicate that within clinically achievable PTX concentrations, the contribution of exosomes to active drug efflux increased with drug concentration and exceeded the p-glycoprotein efflux when the latter was saturated.
Conclusions
Our results indicate (a) chemotherapeutic agents stimulate exosome production or release, and (b) exosome is a mechanism of intercellular drug transfer that contributes to pharmacodynamics of neighboring cells.
中文翻译:

外泌体是细胞间药物转移的机制:定量药理学的应用
目的
外泌体是细胞分泌到细胞外空间的小膜囊泡(直径30-100 nm)。本研究评估了化学治疗剂对外泌体产生和/或释放的影响,并量化了外泌体对细胞间药物转移和药效学的贡献。
方法
用紫杉醇(PTX,2-1000 nM)或阿霉素(DOX,20-1000 nM)处理人类癌细胞(乳腺癌MCF7,从乳房到肺的转移性LM2,卵巢A2780和OVCAR4)治疗24-48小时。使用超速离心从药物处理过的供体细胞(供体细胞)的培养基中分离外泌体,并分析其对乙酰胆碱酯酶的活性,总蛋白,药物浓度以及对未经药物治疗的受体细胞的生物学作用(细胞毒性和抗迁移) (收件人单元格)。这些结果用于建立计算预测定量药理模型。
结果
处于指数生长期的细胞在培养基中释放约220个外泌体/细胞。PTX和DOX以剂量和时间依赖性方式显着促进外泌体的产生和/或释放,对卵巢癌细胞的作用大于对乳腺癌细胞的作用。从供体细胞中分离出的外泌体含有明显的药物水平(2–7 pmole / 10 6用100–1000 nM PTX处理24小时后的细胞),并引起细胞毒性并抑制了受体细胞的迁移。将细胞PTX药代动力学与PTX药效学相结合的定量药理模型成功预测了外泌体对细胞间药物转移的影响,PTX对供体细胞的细胞毒性以及含PTX的外泌体对受体细胞的细胞毒性。附加的模型模拟表明,在临床上可达到的PTX浓度范围内,外泌体对活性药物外排的贡献随着药物浓度的增加而增加,并在p-糖蛋白饱和时超过了p-糖蛋白的外排。
结论
我们的结果表明(a)化学治疗剂刺激外泌体产生或释放,并且(b)外泌体是细胞间药物转移的机制,有助于邻近细胞的药效学。



























 京公网安备 11010802027423号
京公网安备 11010802027423号