当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
RuII-Catalyzed ortho-Sulfonamidation of α-Tetralones with Sulfonyl Azides and Synthesis of Sivelestat by Aromatic C−H Activation
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2017-10-18 07:05:25 , DOI: 10.1002/ajoc.201700356 Medikonda V. Krishna Rao 1 , Keesari Nagarjuna Reddy 1 , Balasubramaniam Sridhar 2 , Basi V. Subba Reddy 1
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2017-10-18 07:05:25 , DOI: 10.1002/ajoc.201700356 Medikonda V. Krishna Rao 1 , Keesari Nagarjuna Reddy 1 , Balasubramaniam Sridhar 2 , Basi V. Subba Reddy 1
Affiliation
|
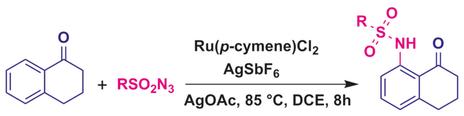
Amide other reactions: A method has been developed for the sulfonamidation of α-tetralones and 1,4-naphthoquinones by using sulfonyl azides and a RuII/AgI catalytic system (see scheme; DCE=1,2-dichloroethane). Tetralones underwent C−H bond functionalization exclusively at the ortho position, whereas 1,4-addition was the primary reaction for 1,4-naphthoquinone. This highly regioselective approach was also used to synthesize sivelestat, an inhibitor of human neutrophil elastase.
中文翻译:

RuII催化磺酰叠氮化物催化α-四氢萘酮的邻氨基磺酰胺化和芳香族CH活化合成Sivelestat
酰胺化其他反应:已经开发了一种方法,该方法通过使用磺酰基叠氮化物和Ru II / Ag I催化体系对α-四氢萘酮和1,4-萘醌进行磺酰胺化(参见方案; DCE = 1,2-二氯乙烷)。Tetralones仅在邻位进行CH键官能化,而1,4-加成是1,4-萘醌的主要反应。这种高度区域选择性的方法也被用来合成人类嗜中性粒细胞弹性蛋白酶抑制剂西乐司他。
更新日期:2017-10-18
中文翻译:

RuII催化磺酰叠氮化物催化α-四氢萘酮的邻氨基磺酰胺化和芳香族CH活化合成Sivelestat
酰胺化其他反应:已经开发了一种方法,该方法通过使用磺酰基叠氮化物和Ru II / Ag I催化体系对α-四氢萘酮和1,4-萘醌进行磺酰胺化(参见方案; DCE = 1,2-二氯乙烷)。Tetralones仅在邻位进行CH键官能化,而1,4-加成是1,4-萘醌的主要反应。这种高度区域选择性的方法也被用来合成人类嗜中性粒细胞弹性蛋白酶抑制剂西乐司他。


























 京公网安备 11010802027423号
京公网安备 11010802027423号