当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Divalent metal ions control activity and inhibition of protein kinases
Metallomics ( IF 3.4 ) Pub Date : 2017-10-04 00:00:00 , DOI: 10.1039/c7mt00204a Matthias J. Knape 1, 2, 3 , Mike Ballez 1, 2, 3 , Nicole C. Burghardt 1, 2, 3 , Bastian Zimmermann 2, 3, 4 , Daniela Bertinetti 1, 2, 3 , Alexandr P. Kornev 5, 6, 7 , Friedrich W. Herberg 1, 2, 3
Metallomics ( IF 3.4 ) Pub Date : 2017-10-04 00:00:00 , DOI: 10.1039/c7mt00204a Matthias J. Knape 1, 2, 3 , Mike Ballez 1, 2, 3 , Nicole C. Burghardt 1, 2, 3 , Bastian Zimmermann 2, 3, 4 , Daniela Bertinetti 1, 2, 3 , Alexandr P. Kornev 5, 6, 7 , Friedrich W. Herberg 1, 2, 3
Affiliation
|
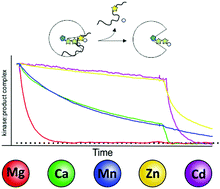
Protein kinases are key enzymes in the regulation of eukaryotic signal transduction. As metalloenzymes they employ divalent cations for catalysis and regulation. We used the catalytic (C) subunit of cAMP-dependent protein kinase (PKA) as a model protein to investigate the role of a variety of physiologically or pathophysiologically relevant divalent metal ions in distinct steps within the catalytic cycle. It is established that divalent metal ions play a crucial role in co-binding of nucleotides and also assist in catalysis. Our studies reveal that besides the physiologically highly relevant magnesium, metals like zinc and manganese can assist in phosphoryl transfer, however, only a few support efficient substrate turnover (turnover catalysis). Those trace metals allow for substrate binding and phosphotransfer but hamper product release. We further established the unique role of magnesium as the common biologically relevant divalent metal ideally enabling (co-) substrate binding and orientation. Magnesium allows stable substrate binding and, on the other hand accelerates product release, thus being extremely efficient in turnover catalysis. We extended our studies to non-catalytic functions of protein kinases looking at pseudokinases, a subfamily of protein kinases inherently lacking critical residues for catalysis. Recently, pseudokinases have been linked to human diseases. Some pseudokinases are still capable of binding metal ions, yet have lost the ability to transfer the phosphoryl group from ATP to a given substrate. Here metal ions stabilize an active like, though catalytically unproductive conformation and are therefore crucial to maintain non-catalytic function. Finally, we demonstrate for the canonical kinase PKA that the trace metal manganese alone can stabilize protein kinases in an active like conformation allowing them to bind substrates even in the absence of nucleotides.
中文翻译:

二价金属离子控制活性和抑制蛋白激酶
蛋白激酶是调节真核信号转导的关键酶。作为金属酶,它们采用二价阳离子进行催化和调节。我们使用cAMP依赖性蛋白激酶(PKA)的催化(C)亚基作为模型蛋白,以研究各种生理或病理生理相关的二价金属离子在催化循环内不同步骤中的作用。已经确定,二价金属离子在核苷酸的共结合中起关键作用,并且还有助于催化。我们的研究表明,除了在生理上具有高度相关性的镁外,锌和锰等金属也可以协助磷酰基转移,但是,只有少数几种支持有效的底物更新(周转催化)。这些痕量金属允许底物结合和磷酸转移,但阻碍了产物的释放。我们进一步确立了镁作为常见的生物学相关二价金属的独特作用,理想地实现了(共)底物的结合和定向。镁可以稳定地与底物结合,另一方面可以加速产品释放,因此在翻转催化方面非常有效。我们将研究扩展到寻找假激酶的蛋白激酶的非催化功能,假激酶是蛋白激酶的一个亚家族,其固有地缺乏关键的催化残基。最近,假激酶已经与人类疾病相关。一些假激酶仍然能够结合金属离子,但失去了将磷酸基团从ATP转移到给定底物的能力。尽管没有催化作用,但金属离子可以稳定类似活性的构象,因此对于维持非催化功能至关重要。最后,
更新日期:2017-10-18
中文翻译:

二价金属离子控制活性和抑制蛋白激酶
蛋白激酶是调节真核信号转导的关键酶。作为金属酶,它们采用二价阳离子进行催化和调节。我们使用cAMP依赖性蛋白激酶(PKA)的催化(C)亚基作为模型蛋白,以研究各种生理或病理生理相关的二价金属离子在催化循环内不同步骤中的作用。已经确定,二价金属离子在核苷酸的共结合中起关键作用,并且还有助于催化。我们的研究表明,除了在生理上具有高度相关性的镁外,锌和锰等金属也可以协助磷酰基转移,但是,只有少数几种支持有效的底物更新(周转催化)。这些痕量金属允许底物结合和磷酸转移,但阻碍了产物的释放。我们进一步确立了镁作为常见的生物学相关二价金属的独特作用,理想地实现了(共)底物的结合和定向。镁可以稳定地与底物结合,另一方面可以加速产品释放,因此在翻转催化方面非常有效。我们将研究扩展到寻找假激酶的蛋白激酶的非催化功能,假激酶是蛋白激酶的一个亚家族,其固有地缺乏关键的催化残基。最近,假激酶已经与人类疾病相关。一些假激酶仍然能够结合金属离子,但失去了将磷酸基团从ATP转移到给定底物的能力。尽管没有催化作用,但金属离子可以稳定类似活性的构象,因此对于维持非催化功能至关重要。最后,


























 京公网安备 11010802027423号
京公网安备 11010802027423号