当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tyr25, Tyr58 and Trp133 of Escherichia coli bacterioferritin transfer electrons between iron in the central cavity and the ferroxidase centre
Metallomics ( IF 3.4 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1039/c7mt00187h Justin M. Bradley 1, 2, 3, 4, 5 , Dimitri A. Svistunenko 6, 7, 8, 9, 10 , Geoffrey R. Moore 1, 2, 3, 4, 5 , Nick E. Le Brun 1, 2, 3, 4, 5
Metallomics ( IF 3.4 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1039/c7mt00187h Justin M. Bradley 1, 2, 3, 4, 5 , Dimitri A. Svistunenko 6, 7, 8, 9, 10 , Geoffrey R. Moore 1, 2, 3, 4, 5 , Nick E. Le Brun 1, 2, 3, 4, 5
Affiliation
|
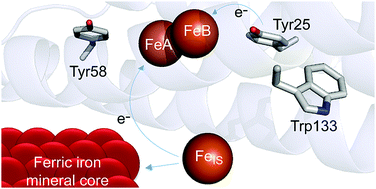
Ferritins are 24meric proteins that overcome problems of toxicity, insolubility and poor bioavailability of iron in all types of cells by storing it in the form of a ferric mineral within their central cavities. In the bacterioferritin (BFR) from Escherichia coli iron mineralization kinetics have been shown to be dependent on an intra-subunit catalytic diiron cofactor site (the ferroxidase centre), three closely located aromatic residues and an inner surface iron site. One of the aromatic residues, Tyr25, is the site of formation of a transient radical, but the roles of the other two residues, Tyr58 and Trp133, are unknown. Here we show that these residues are important for the rates of formation and decay of the Tyr25 radical and decay of a secondary radical observed during Tyr25 radical decay. The data support a mechanism in which these aromatic residues function in electron transfer from the inner surface site to the ferroxidase centre.
中文翻译:

大肠杆菌细菌铁蛋白的Tyr25,Tyr58和Trp133在中心腔中的铁与亚铁氧化酶中心之间转移电子
铁蛋白是由24种蛋白质组成的蛋白质,通过将铁以铁矿物质的形式存储在其中心腔内,从而克服了铁在所有类型细胞中的毒性,不溶性和不良生物利用度的问题。来自大肠杆菌的细菌铁蛋白(BFR)铁的矿化动力学已显示出取决于亚基内催化二铁辅因子位点(亚铁氧化酶中心),三个位置紧密的芳族残基和一个内表面铁位点。芳香族残基之一Tyr25是瞬态自由基的形成位点,但其他两个残基Tyr58和Trp133的作用尚不清楚。在这里,我们表明这些残基对于Tyr25自由基的形成和衰变速率以及在Tyr25自由基衰变期间观察到的第二自由基的衰变率都很重要。数据支持了一种机制,其中这些芳族残基在电子从内表面位点转移至亚铁氧化酶中心的过程中起作用。
更新日期:2017-10-18
中文翻译:

大肠杆菌细菌铁蛋白的Tyr25,Tyr58和Trp133在中心腔中的铁与亚铁氧化酶中心之间转移电子
铁蛋白是由24种蛋白质组成的蛋白质,通过将铁以铁矿物质的形式存储在其中心腔内,从而克服了铁在所有类型细胞中的毒性,不溶性和不良生物利用度的问题。来自大肠杆菌的细菌铁蛋白(BFR)铁的矿化动力学已显示出取决于亚基内催化二铁辅因子位点(亚铁氧化酶中心),三个位置紧密的芳族残基和一个内表面铁位点。芳香族残基之一Tyr25是瞬态自由基的形成位点,但其他两个残基Tyr58和Trp133的作用尚不清楚。在这里,我们表明这些残基对于Tyr25自由基的形成和衰变速率以及在Tyr25自由基衰变期间观察到的第二自由基的衰变率都很重要。数据支持了一种机制,其中这些芳族残基在电子从内表面位点转移至亚铁氧化酶中心的过程中起作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号