Journal of Controlled Release ( IF 10.8 ) Pub Date : 2017-10-17 , DOI: 10.1016/j.jconrel.2017.10.016 Shaaban A. Mousa , Mohammed Shaqura , Mohammed Al-Madol , Sascha Tafelski , Baled I. Khalefa , Mehdi Shakibaei , Michael Schäfer
|
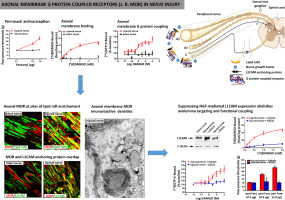
The mechanisms of axonal trafficking and membrane targeting are well established for sodium channels, which are the principle targets for perineurally applied local anaesthetics. However, they have not been thoroughly investigated for G protein coupled receptors such as mu-opioid receptors (MOR). Focusing on these axonal mechanisms, we found that axonal MOR functionality is quite distinct in two different pain states, i.e. hindpaw inflammation and nerve injury. We observed axonal membrane MOR binding and functional G protein coupling exclusively at sites of CCI nerve injury. Moreover at these axonal membrane sites, MOR exhibited extensive co-localization with the membrane proteins SNAP and Na/K-ATPase as well as NGF-dependent enhanced lipid rafts and L1CAM anchoring proteins. Silencing endogenous L1CAM with intrathecal L1CAM specific siRNA, disrupting lipid rafts with the perineurial cholesterol-sequestering agent MβCD, as well as suppressing NGF receptor activation with the perineurial NGF receptor inhibitor K252a abrogated MOR axonal membrane integration, functional coupling, and agonist-elicited antinociception at sites of nerve injury. These findings suggest that local conceptual changes resulting from nerve injury are required for the establishment of functional axonal membrane MOR. Axonal integration and subsequent accessibility of functionally coupled MOR are of great relevance particularly for patients suffering from severe pain due to nerve injury or tumour infiltration.
中文翻译:

轴突G蛋白偶联mu阿片受体的可及性要求改变轴突膜靶向性以调节疼痛
钠通道已经确立了轴突运输和膜靶向的机制,钠通道是会阴部应用局部麻醉剂的主要目标。但是,尚未对G蛋白偶联受体(例如mu阿片类受体(MOR))进行彻底研究。着眼于这些轴突机制,我们发现轴突MOR功能在两种不同的疼痛状态(即后爪发炎和神经损伤)中非常不同。我们观察到仅在CCI神经损伤的部位观察到轴突膜MOR结合和功能性G蛋白偶联。此外,在这些轴突膜位点,MOR与膜蛋白SNAP和Na / K-ATPase以及NGF依赖性增强型脂筏和L1CAM锚定蛋白广泛共定位。用鞘内L1CAM特异的siRNA沉默内源性L1CAM,用会尿的胆固醇替代剂MβCD破坏脂质筏,并用会尿的NGF受体抑制剂K252a抑制NGF受体活化,消除了MOR在神经损伤部位的轴突膜整合,功能偶联和激动剂引起的抗伤害感受。这些发现表明,功能性轴突膜MOR的建立需要神经损伤引起的局部概念改变。轴突整合以及功能性MOR的后续可及性具有重大意义,特别是对于因神经损伤或肿瘤浸润而遭受严重疼痛的患者。以及用神经周围神经生长因子受体抑制剂K252a抑制NGF受体激活,可消除MOR轴突膜的整合,功能偶联以及激动剂引起的神经损伤部位的伤害感受。这些发现表明,功能性轴突膜MOR的建立需要神经损伤引起的局部概念改变。轴突整合以及功能性MOR的后续可及性具有重大意义,特别是对于因神经损伤或肿瘤浸润而遭受严重疼痛的患者。以及用神经周围神经生长因子受体抑制剂K252a抑制NGF受体激活,可消除MOR轴突膜的整合,功能偶联以及激动剂引起的神经损伤部位的伤害感受。这些发现表明,功能性轴突膜MOR的建立需要神经损伤引起的局部概念改变。轴突整合以及功能性MOR的后续可及性具有重大意义,特别是对于因神经损伤或肿瘤浸润而遭受严重疼痛的患者。



























 京公网安备 11010802027423号
京公网安备 11010802027423号