Journal of Controlled Release ( IF 10.8 ) Pub Date : 2017-10-16 , DOI: 10.1016/j.jconrel.2017.10.024 Tong-Fei Li , Ke Li , Chao Wang , Xin Liu , Yu Wen , Yong-Hong Xu , Quan Zhang , Qiu-Ya Zhao , Ming Shao , Yan-Ze Li , Min Han , Naoki Komatsu , Li Zhao , Xiao Chen
|
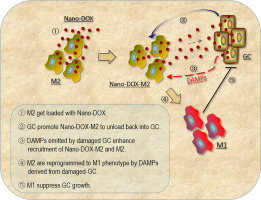
Glioblastoma (GBM) is the most frequent and malignant brain tumor with a high mortality rate. The presence of a large population of macrophages (Mφ) in the tumor microenvironment is a prominent feature of GBM and these so-called tumor-associated Mφ (TAM) closely interact with the GBM cells to promote the survival, progression and therapy resistance of the GBM. Various therapeutic strategies have been devised either targeting the GBM cells or the TAM but few have addressed the cross-talks between the two cell populations. The present study was carried out to explore the possibility of exploiting the cross-talks between the GBM cells (GC) and TAM for modulation of the GBM microenvironment through using Nano-DOX, a drug composite based on nanodiamonds bearing doxorubicin. In the in vitro work on human cell models, Nano-DOX-loaded TAM were first shown to be viable and able to infiltrate three-dimensional GC spheroids and release cargo drug therein. GC were then demonstrated to encourage Nano-DOX-loaded TAM to unload Nano-DOX back into GC which consequently emitted damage-associated molecular patterns (DAMPs) that are powerful immunostimulatory agents as well as indicators of cell damage. Nano-DOX was next proven to be a more potent inducer of GC DAMPs emission than doxorubicin. As a result, Nano-DOX-damaged GC exhibited an enhanced ability to attract both TAM and Nano-DOX-loaded TAM. Most remarkably, Nano-DOX-damaged GC reprogrammed the TAM from a pro-GBM phenotype to an anti-GBM phenotype that suppressed GC growth. Finally, the in vivo relevance of the in vitro findings was tested in animal study. Mice bearing orthotopic human GBM xenografts were intravenously injected with Nano-DOX-loaded mouse TAM which were found releasing drug in the GBM xenografts 24 h after injection. GC damage was evidenced by the induction of DAMPs emission within the xenografts and a shift of TAM phenotype was detected as well. Taken together, our results demonstrate a novel way with therapeutic potential to harness the cross-talk between GBM cells and TAM for modulation of the tumor immune microenvironment.
中文翻译:

利用纳米药物利用胶质母细胞瘤免疫微环境调节肿瘤细胞与肿瘤相关巨噬细胞之间的相互作用
胶质母细胞瘤(GBM)是最常见,最恶性的脑肿瘤,死亡率很高。肿瘤微环境中大量巨噬细胞(Mφ)的存在是GBM的突出特征,这些所谓的肿瘤相关Mφ(TAM)与GBM细胞紧密相互作用,从而促进了巨噬细胞的存活,进展和治疗耐药性GBM。已经设计出了针对GBM细胞或TAM的多种治疗策略,但是很少涉及两种细胞群之间的串扰。进行本研究以探索通过使用基于带有阿霉素的纳米金刚石的药物复合物Nano-DOX来利用GBM细胞(GC)和TAM之间的串扰来调节GBM微环境的可能性。在人体细胞模型的体外研究中,首次显示了装载纳米DOX的TAM是可行的,并且能够渗透三维GC球体并在其中释放货运药物。然后证明了GC可以鼓励装载Nano-DOX的TAM将Nano-DOX卸载回GC,从而释放出与损伤相关的分子模式(DAMP),它们是强大的免疫刺激剂以及细胞损伤的指标。下一步,Nano-DOX被证明比阿霉素更有效地诱导了GC DAMPs的排放。结果,损坏了Nano-DOX的GC表现出了更高的吸引TAM和装载Nano-DOX的TAM的能力。最显着的是,Nano-DOX损坏的GC将TAM从前GBM表型重新编程为抑制GC生长的抗GBM表型。最后,在动物研究中测试了体外发现的体内相关性。向携带原位人GBM异种移植物的小鼠静脉内注射Nano-DOX负载的小鼠TAM,发现小鼠在注射后24小时内在GBM异种移植物中释放药物。通过在异种移植物中诱导DAMPs发射来证明GC损伤,并且还检测到TAM表型的转变。综上所述,我们的结果证明了一种具有治疗潜力的新方法,可以利用GBM细胞和TAM之间的串扰来调节肿瘤免疫微环境。



























 京公网安备 11010802027423号
京公网安备 11010802027423号