当前位置:
X-MOL 学术
›
PLOS Pathog.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
TRIM5α SPRY/coiled-coil interactions optimize avid retroviral capsid recognition.
PLoS Pathogens ( IF 6.7 ) Pub Date : 2017-10-17 , DOI: 10.1371/journal.ppat.1006686 Marcin D Roganowicz 1 , Sevnur Komurlu 2 , Santanu Mukherjee 2 , Jacek Plewka 1 , Steven L Alam 3 , Katarzyna A Skorupka 1 , Yueping Wan 1 , Damian Dawidowski 4 , David S Cafiso 4 , Barbie K Ganser-Pornillos 1 , Edward M Campbell 2 , Owen Pornillos 1
PLoS Pathogens ( IF 6.7 ) Pub Date : 2017-10-17 , DOI: 10.1371/journal.ppat.1006686 Marcin D Roganowicz 1 , Sevnur Komurlu 2 , Santanu Mukherjee 2 , Jacek Plewka 1 , Steven L Alam 3 , Katarzyna A Skorupka 1 , Yueping Wan 1 , Damian Dawidowski 4 , David S Cafiso 4 , Barbie K Ganser-Pornillos 1 , Edward M Campbell 2 , Owen Pornillos 1
Affiliation
|
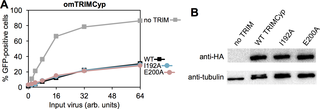
Restriction factors are important components of intrinsic cellular defense mechanisms against viral pathogens. TRIM5α is a restriction factor that intercepts the incoming capsid cores of retroviruses such as HIV and provides an effective species-specific barrier to retroviral infection. The TRIM5α SPRY domain directly binds the capsid with only very weak, millimolar-level affinity, and productive capsid recognition therefore requires both TRIM5α dimerization and assembly of the dimers into a multivalent hexagonal lattice to promote avid binding. Here, we explore the important unresolved question of whether the SPRY domains are flexibly linked to the TRIM lattice or more precisely positioned to maximize avidity. Biochemical and biophysical experiments indicate that the linker segment connecting the SPRY domain to the coiled-coil domain adopts an α-helical fold, and that this helical portion mediates interactions between the two domains. Targeted mutations were generated to disrupt the putative packing interface without affecting dimerization or higher-order assembly, and we identified mutant proteins that were nevertheless deficient in capsid binding in vitro and restriction activity in cells. Our studies therefore support a model wherein substantial avidity gains during assembly-mediated capsid recognition by TRIM5α come in part from tailored spacing of tethered recognition domains.
中文翻译:

TRIM5α SPRY/卷曲螺旋相互作用优化了狂热的逆转录病毒衣壳识别。
限制因子是针对病毒病原体的内在细胞防御机制的重要组成部分。TRIM5α 是一种限制因子,可拦截 HIV 等逆转录病毒的进入衣壳核心,并为逆转录病毒感染提供有效的物种特异性屏障。TRIM5α SPRY 结构域仅以非常弱的毫摩尔级亲和力直接结合衣壳,并且有效的衣壳识别因此需要 TRIM5α 二聚化和二聚体组装成多价六边形晶格以促进亲和性结合。在这里,我们探讨了一个重要的未解决问题,即 SPRY 域是否灵活地连接到 TRIM 晶格或更精确地定位以最大化亲和力。生化和生物物理实验表明,将 SPRY 结构域连接到卷曲螺旋结构域的接头片段采用 α-螺旋折叠,并且该螺旋部分介导了两个结构域之间的相互作用。产生靶向突变以破坏假定的包装界面而不影响二聚化或高阶组装,并且我们鉴定了在体外衣壳结合和细胞中的限制活性方面仍然存在缺陷的突变蛋白。因此,我们的研究支持一个模型,其中在 TRIM5α 组装介导的衣壳识别过程中显着的亲和力增益部分来自系留识别域的定制间距。产生靶向突变以破坏假定的包装界面而不影响二聚化或高阶组装,并且我们鉴定了在体外衣壳结合和细胞中的限制活性方面仍然存在缺陷的突变蛋白。因此,我们的研究支持一个模型,其中在 TRIM5α 组装介导的衣壳识别过程中,大量的亲和力增益部分来自系留识别域的定制间距。产生靶向突变以破坏假定的包装界面而不影响二聚化或高阶组装,并且我们鉴定了在体外衣壳结合和细胞中的限制活性方面仍然存在缺陷的突变蛋白。因此,我们的研究支持一个模型,其中在 TRIM5α 组装介导的衣壳识别过程中显着的亲和力增益部分来自系留识别域的定制间距。
更新日期:2017-10-17
中文翻译:

TRIM5α SPRY/卷曲螺旋相互作用优化了狂热的逆转录病毒衣壳识别。
限制因子是针对病毒病原体的内在细胞防御机制的重要组成部分。TRIM5α 是一种限制因子,可拦截 HIV 等逆转录病毒的进入衣壳核心,并为逆转录病毒感染提供有效的物种特异性屏障。TRIM5α SPRY 结构域仅以非常弱的毫摩尔级亲和力直接结合衣壳,并且有效的衣壳识别因此需要 TRIM5α 二聚化和二聚体组装成多价六边形晶格以促进亲和性结合。在这里,我们探讨了一个重要的未解决问题,即 SPRY 域是否灵活地连接到 TRIM 晶格或更精确地定位以最大化亲和力。生化和生物物理实验表明,将 SPRY 结构域连接到卷曲螺旋结构域的接头片段采用 α-螺旋折叠,并且该螺旋部分介导了两个结构域之间的相互作用。产生靶向突变以破坏假定的包装界面而不影响二聚化或高阶组装,并且我们鉴定了在体外衣壳结合和细胞中的限制活性方面仍然存在缺陷的突变蛋白。因此,我们的研究支持一个模型,其中在 TRIM5α 组装介导的衣壳识别过程中显着的亲和力增益部分来自系留识别域的定制间距。产生靶向突变以破坏假定的包装界面而不影响二聚化或高阶组装,并且我们鉴定了在体外衣壳结合和细胞中的限制活性方面仍然存在缺陷的突变蛋白。因此,我们的研究支持一个模型,其中在 TRIM5α 组装介导的衣壳识别过程中,大量的亲和力增益部分来自系留识别域的定制间距。产生靶向突变以破坏假定的包装界面而不影响二聚化或高阶组装,并且我们鉴定了在体外衣壳结合和细胞中的限制活性方面仍然存在缺陷的突变蛋白。因此,我们的研究支持一个模型,其中在 TRIM5α 组装介导的衣壳识别过程中显着的亲和力增益部分来自系留识别域的定制间距。



























 京公网安备 11010802027423号
京公网安备 11010802027423号