当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Towards sustainable manufacture of epichlorohydrin from glycerol using hydrotalcite-derived basic oxides
Green Chemistry ( IF 9.8 ) Pub Date : 2017-10-16 00:00:00 , DOI: 10.1039/c7gc02610b Giacomo M. Lari 1, 2, 3, 4, 5 , Giorgio Pastore 1, 2, 3, 4, 5 , Cecilia Mondelli 1, 2, 3, 4, 5 , Javier Pérez-Ramírez 1, 2, 3, 4, 5
Green Chemistry ( IF 9.8 ) Pub Date : 2017-10-16 00:00:00 , DOI: 10.1039/c7gc02610b Giacomo M. Lari 1, 2, 3, 4, 5 , Giorgio Pastore 1, 2, 3, 4, 5 , Cecilia Mondelli 1, 2, 3, 4, 5 , Javier Pérez-Ramírez 1, 2, 3, 4, 5
Affiliation
|
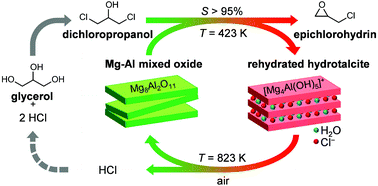
Commercial two-step processes to convert glycerol into epichlorohydrin are more benign compared to the predominant industrial route starting from propene in terms of materials requirements and CO2 emissions. Still, the use of alkali hydroxides in stoichiometric amounts in the second reaction, i.e., the dehydrochlorination of the dichloropropanol intermediate, leads to the formation of large amounts of salt wastes, thus limiting the greenness of the technology. Here, we show for the first time that the latter transformation can be selectively conducted in the gas phase in the presence of a heterogeneous hydrotalcite-derived mixed oxide of Al and Mg. Upon reaction, the lamellar solid is rehydrated to a hydrotalcite-like compound, which can effectively activate the alcoholic group of dichloropropanol owing to its strong Brønsted basic character and moderately high surface area. In-depth characterisation of the porous, compositional, structural and acid/base properties demonstrates that the HCl formed during the reaction causes the progressive exchange of interlayer OH groups by Cl atoms, thus gradually diminishing the reactivity of the material. Facile calcination restores the original mixed oxide structure and is shown to enable three equivalent consecutive reaction runs. Since the HCl evolved along with water upon regeneration can be recycled in the first step of the process, i.e., glycerol hydrochlorination, our approach paves the way for a waste-free and more atom efficient biobased epichlorohydrin production process.
中文翻译:

使用水滑石衍生的碱性氧化物实现甘油的可持续生产环氧氯丙烷
与从丙烯开始的主要工业路线相比,就材料需求和CO 2排放而言,将甘油转化为环氧氯丙烷的商业两步工艺更为温和。另外,在第二反应中使用化学计量的碱金属氢氧化物,即二氯丙醇中间体的脱氯化氢导致大量盐分的形成,从而限制了该技术的绿色环保。在此,我们首次表明,在存在水滑石衍生的Al和Mg混合氧化物的情况下,可以在气相中选择性地进行后者的转化。反应后,层状固体再水化为类水滑石,由于其强的布朗斯台德碱性和适度的高表面积,可有效活化二氯丙醇的醇基。对多孔,组成,结构和酸/碱性质的深入表征表明,反应期间形成的HCl会导致Cl原子逐步交换层间OH基,因此逐渐降低了材料的反应性。容易的煅烧恢复了原始的混合氧化物结构,并且显示出能够进行三个相等的连续反应运行。由于再生过程中与水一起释放出的HCl可以在流程的第一步中回收利用,即甘油氢氯化,我们的方法为无浪费和原子效率更高的生物基环氧氯丙烷生产工艺铺平了道路。
更新日期:2018-01-02
中文翻译:

使用水滑石衍生的碱性氧化物实现甘油的可持续生产环氧氯丙烷
与从丙烯开始的主要工业路线相比,就材料需求和CO 2排放而言,将甘油转化为环氧氯丙烷的商业两步工艺更为温和。另外,在第二反应中使用化学计量的碱金属氢氧化物,即二氯丙醇中间体的脱氯化氢导致大量盐分的形成,从而限制了该技术的绿色环保。在此,我们首次表明,在存在水滑石衍生的Al和Mg混合氧化物的情况下,可以在气相中选择性地进行后者的转化。反应后,层状固体再水化为类水滑石,由于其强的布朗斯台德碱性和适度的高表面积,可有效活化二氯丙醇的醇基。对多孔,组成,结构和酸/碱性质的深入表征表明,反应期间形成的HCl会导致Cl原子逐步交换层间OH基,因此逐渐降低了材料的反应性。容易的煅烧恢复了原始的混合氧化物结构,并且显示出能够进行三个相等的连续反应运行。由于再生过程中与水一起释放出的HCl可以在流程的第一步中回收利用,即甘油氢氯化,我们的方法为无浪费和原子效率更高的生物基环氧氯丙烷生产工艺铺平了道路。



























 京公网安备 11010802027423号
京公网安备 11010802027423号