当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Novel tetrahydroacridine derivatives with iodobenzoic acid moiety as multifunctional acetylcholinesterase inhibitors
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2017-10-24 , DOI: 10.1111/cbdd.13111 Robert Skibiński 1 , Kamila Czarnecka 2 , Małgorzata Girek 2 , Ireneusz Bilichowski 2 , Nina Chufarova 2 , Elżbieta Mikiciuk-Olasik 2 , Paweł Szymański 2
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2017-10-24 , DOI: 10.1111/cbdd.13111 Robert Skibiński 1 , Kamila Czarnecka 2 , Małgorzata Girek 2 , Ireneusz Bilichowski 2 , Nina Chufarova 2 , Elżbieta Mikiciuk-Olasik 2 , Paweł Szymański 2
Affiliation
|
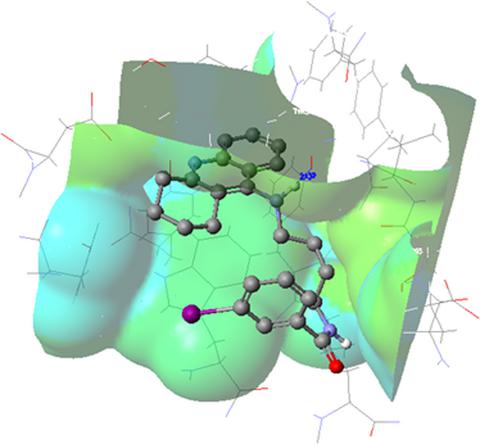
New synthesized series of 9‐amino‐1,2,3,4‐tetrahydroacridine derivatives with iodobenzoic acid moiety were studied for their inhibitory activity toward cholinesterase and against β‐amyloid aggregation. All novel molecules 3a–3i interacted with both cholinesterases—acetylcholinesterase and butyrylcholinesterase—delivered nanomolar IC50 values. The structure–activity relationship showed that N‐butyl moiety derivatives are stronger inhibitors toward AChE and BuChE than N‐ethyl and N‐propyl moieties compounds. The most potent compound toward acetylcholinesterase was inhibitor 3f (IC50 = 31.2 nm), and it was more active than reference drug, tacrine (IC50 = 100.2 nm). Compound 3f showed strong inhibition of butyrylcholinesterase (IC50 = 8.0 nm), also higher than tacrine (IC50 = 16.3 nm). In the kinetic studies, compound 3f revealed mixed type of acetylcholinesterase inhibition. The computer modeling was carried out. The most active compound 3f was confirmed as peripheral anionic site inhibitor of acetylcholinesterase. Moreover, molecule 3f inhibited β‐amyloid aggregation (at the concentration 10 μm—24.96% of inhibition, 25 μm—72%, 50 μm—78.44%, and 100 μm—84.92%). Therefore, among all examined, compound 3f is the most promising molecule for further, more detailed research of novel multifunctional agents in the therapy of Alzheimer's disease.
中文翻译:

具有碘苯甲酸部分的新型四氢ac啶衍生物作为多功能乙酰胆碱酯酶抑制剂
研究了新合成的具有碘苯甲酸部分的9-氨基1,2,3,4-四氢ac啶衍生物系列对胆碱酯酶和β-淀粉样蛋白聚集的抑制活性。所有新型分子3a-3i与胆碱酯酶(乙酰胆碱酯酶和丁酰胆碱酯酶)都相互作用,可提供纳摩尔IC 50值。结构-活性关系表明,Ñ丁基部分衍生物是朝向乙酰胆碱酯酶和丁酰胆碱酯酶抑制剂更强比ñ -乙基和ñ -丙基部分的化合物。对乙酰胆碱酯酶最有效的化合物是抑制剂3f(IC 50 = 31.2 n m),并且比参考药物他克林(IC 50 = 100.2 n m)具有更高的活性。化合物3f对丁酰胆碱酯酶(IC 50 = 8.0 n m)表现出强烈的抑制作用,也比他克林(IC 50 = 16.3 n m)高。在动力学研究中,化合物3f显示出混合类型的乙酰胆碱酯酶抑制作用。进行了计算机建模。活性最高的化合物3f被确认为乙酰胆碱酯酶的外围阴离子位点抑制剂。此外,分子3F抑制β淀粉样蛋白聚集(在浓度为10μ米-24.96抑制%,25μ米-72%,50μ米-78.44%和100μ米-84.92%)。因此,在所有检查的化合物中,化合物3f是用于治疗阿尔茨海默氏病的新型多功能药物的进一步,更详细研究的最有前途的分子。
更新日期:2017-10-24
中文翻译:

具有碘苯甲酸部分的新型四氢ac啶衍生物作为多功能乙酰胆碱酯酶抑制剂
研究了新合成的具有碘苯甲酸部分的9-氨基1,2,3,4-四氢ac啶衍生物系列对胆碱酯酶和β-淀粉样蛋白聚集的抑制活性。所有新型分子3a-3i与胆碱酯酶(乙酰胆碱酯酶和丁酰胆碱酯酶)都相互作用,可提供纳摩尔IC 50值。结构-活性关系表明,Ñ丁基部分衍生物是朝向乙酰胆碱酯酶和丁酰胆碱酯酶抑制剂更强比ñ -乙基和ñ -丙基部分的化合物。对乙酰胆碱酯酶最有效的化合物是抑制剂3f(IC 50 = 31.2 n m),并且比参考药物他克林(IC 50 = 100.2 n m)具有更高的活性。化合物3f对丁酰胆碱酯酶(IC 50 = 8.0 n m)表现出强烈的抑制作用,也比他克林(IC 50 = 16.3 n m)高。在动力学研究中,化合物3f显示出混合类型的乙酰胆碱酯酶抑制作用。进行了计算机建模。活性最高的化合物3f被确认为乙酰胆碱酯酶的外围阴离子位点抑制剂。此外,分子3F抑制β淀粉样蛋白聚集(在浓度为10μ米-24.96抑制%,25μ米-72%,50μ米-78.44%和100μ米-84.92%)。因此,在所有检查的化合物中,化合物3f是用于治疗阿尔茨海默氏病的新型多功能药物的进一步,更详细研究的最有前途的分子。

























 京公网安备 11010802027423号
京公网安备 11010802027423号