Applied Catalysis A: General ( IF 5.5 ) Pub Date : 2017-09-29 , DOI: 10.1016/j.apcata.2017.09.036 Janina Okal , Mirosław Zawadzki , Piotr Kraszkiewicz , Katarzyna Adamska
|
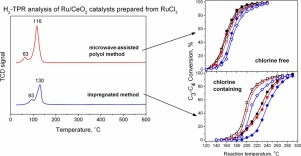
Two preparation methods and two metal salt precursors (chlorine containing and chlorine-free) were used to synthesize Ru/CeO2 catalysts and their catalytic behaviour has been evaluated in combustion of propane, n-butane and isobutane mixture. In the first method, Ru nanoparticles (Ru NPs) deposited on CeO2 were synthesized by a microwave-assisted polyol method using RuCl3 as a metal precursor and ethylene glycol as a reducing agent. In the second, Ru/CeO2 catalysts were prepared by traditional incipient wetness impregnation method using RuCl3 or Ru(NO)(NO3)3. ICP-AES, N2 adsorption-desorption, H2-TPR, XRD, HRTEM and SEM-EDS techniques were used to characterize the catalysts. In all the Ru/CeO2 catalysts only highly dispersed Ru nanoparticles (1–2 nm) were present. The catalytic data show that the preparation method and the nature of the metal precursor have a significant influence on catalytic performance of the Ru/CeO2 catalysts. The VOC reactivity order (iso-butane > n-butane > propane) show that propane is the most difficult to oxidize. The Ru(NPs)/CeO2 and impregnated Ru(N)/CeO2 catalyst exhibited much higher activity and stability than that of impregnated Ru(Cl)/CeO2. It was found that the Ru(NPs)/CeO2 nano-catalyst was free of chlorine species although it had been prepared from RuCl3. The differences in the activity have been ascribed to the presence of Cl− ions on the Ru(Cl)/CeO2 catalyst and to much better reducibility of the chlorine-free Ru(NPs) and Ru(N) catalysts. The Cl species have a negative impact on the redox properties of CeO2, they have some influence on Ru agglomeration during long-term activity tests (50 h), and consequently, the chlorinated catalyst exhibited a poorer activity and stability compared with those of the Cl-free catalysts.
中文翻译:

用于轻烃混合物燃烧的Ru / CeO 2催化剂:制备方法和金属盐前体的影响
用两种制备方法和两种金属盐前体(含氯和无氯)合成Ru / CeO 2催化剂,并在丙烷,正丁烷和异丁烷混合物的燃烧中评估了它们的催化性能。在第一种方法中,以RuCl 3为金属前体,以乙二醇为还原剂,通过微波辅助多元醇法合成了沉积在CeO 2上的Ru纳米颗粒(Ru NPs)。其次,采用RuCl 3或Ru(NO)(NO 3)3通过传统的湿法浸渍法制备了Ru / CeO 2催化剂。ICP-AES,N 2吸附-解吸,H使用2- TPR,XRD,HRTEM和SEM-EDS技术表征催化剂。在所有Ru / CeO 2催化剂中,仅存在高度分散的Ru纳米颗粒(1-2 nm)。催化数据表明,金属前驱体的制备方法和性质对Ru / CeO 2催化剂的催化性能有重要影响。VOC的反应顺序(异丁烷>正丁烷>丙烷)显示丙烷是最难氧化的。与浸渍Ru(Cl)/ CeO 2相比,Ru(NPs)/ CeO 2和浸渍Ru(N)/ CeO 2催化剂具有更高的活性和稳定性。发现Ru(NPs)/ CeO 2尽管它是由RuCl 3制备的,但纳米催化剂不含氯。在活动的差异已经被归因于氯的存在-离子在Ru(Cl)的/的CeO 2催化剂,并在无氯钌(NPS)和Ru(N)的催化剂的更好的还原性。Cl种类对CeO 2的氧化还原性能有负面影响,在长期活性试验(50 h)中它们对Ru的团聚有一定影响,因此,氯化的催化剂与CeO 2相比,具有较差的活性和稳定性。无氯催化剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号