当前位置:
X-MOL 学术
›
Cancer Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Coordinated Splicing of Regulatory Detained Introns within Oncogenic Transcripts Creates an Exploitable Vulnerability in Malignant Glioma.
Cancer Cell ( IF 50.3 ) Pub Date : 2017-10-09 , DOI: 10.1016/j.ccell.2017.08.018 Christian J Braun 1 , Monica Stanciu 1 , Paul L Boutz 1 , Jesse C Patterson 1 , David Calligaris 2 , Fumi Higuchi 3 , Rachit Neupane 1 , Silvia Fenoglio 1 , Daniel P Cahill 3 , Hiroaki Wakimoto 3 , Nathalie Y R Agar 4 , Michael B Yaffe 5 , Phillip A Sharp 1 , Michael T Hemann 1 , Jacqueline A Lees 1
Cancer Cell ( IF 50.3 ) Pub Date : 2017-10-09 , DOI: 10.1016/j.ccell.2017.08.018 Christian J Braun 1 , Monica Stanciu 1 , Paul L Boutz 1 , Jesse C Patterson 1 , David Calligaris 2 , Fumi Higuchi 3 , Rachit Neupane 1 , Silvia Fenoglio 1 , Daniel P Cahill 3 , Hiroaki Wakimoto 3 , Nathalie Y R Agar 4 , Michael B Yaffe 5 , Phillip A Sharp 1 , Michael T Hemann 1 , Jacqueline A Lees 1
Affiliation
|
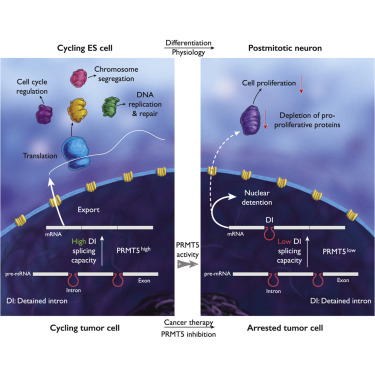
Glioblastoma (GBM) is a devastating malignancy with few therapeutic options. We identify PRMT5 in an in vivo GBM shRNA screen and show that PRMT5 knockdown or inhibition potently suppresses in vivo GBM tumors, including patient-derived xenografts. Pathway analysis implicates splicing in cellular PRMT5 dependency, and we identify a biomarker that predicts sensitivity to PRMT5 inhibition. We find that PRMT5 deficiency primarily disrupts the removal of detained introns (DIs). This impaired DI splicing affects proliferation genes, whose downregulation coincides with cell cycle defects, senescence and/or apoptosis. We further show that DI programs are evolutionarily conserved and operate during neurogenesis, suggesting that they represent a physiological regulatory mechanism. Collectively, these findings reveal a PRMT5-regulated DI-splicing program as an exploitable cancer vulnerability.
中文翻译:

致癌转录本中调节性滞留内含子的协调剪接在恶性胶质瘤中产生了可利用的漏洞。
胶质母细胞瘤(GBM)是一种毁灭性的恶性肿瘤,治疗选择很少。我们在体内 GBM shRNA 筛选中鉴定了 PRMT5,并表明 PRMT5 敲低或抑制可有效抑制体内 GBM 肿瘤,包括患者来源的异种移植物。通路分析表明剪接与细胞 PRMT5 依赖性相关,我们鉴定了一种预测对 PRMT5 抑制敏感性的生物标志物。我们发现 PRMT5 缺陷主要破坏了滞留内含子 (DI) 的去除。这种受损的 DI 剪接会影响增殖基因,其下调与细胞周期缺陷、衰老和/或凋亡同时发生。我们进一步表明,DI 程序在进化上是保守的,并且在神经发生过程中起作用,这表明它们代表了一种生理调节机制。总的来说,这些发现揭示了 PRMT5 调节的 DI 剪接程序是一种可利用的癌症漏洞。
更新日期:2017-09-29
中文翻译:

致癌转录本中调节性滞留内含子的协调剪接在恶性胶质瘤中产生了可利用的漏洞。
胶质母细胞瘤(GBM)是一种毁灭性的恶性肿瘤,治疗选择很少。我们在体内 GBM shRNA 筛选中鉴定了 PRMT5,并表明 PRMT5 敲低或抑制可有效抑制体内 GBM 肿瘤,包括患者来源的异种移植物。通路分析表明剪接与细胞 PRMT5 依赖性相关,我们鉴定了一种预测对 PRMT5 抑制敏感性的生物标志物。我们发现 PRMT5 缺陷主要破坏了滞留内含子 (DI) 的去除。这种受损的 DI 剪接会影响增殖基因,其下调与细胞周期缺陷、衰老和/或凋亡同时发生。我们进一步表明,DI 程序在进化上是保守的,并且在神经发生过程中起作用,这表明它们代表了一种生理调节机制。总的来说,这些发现揭示了 PRMT5 调节的 DI 剪接程序是一种可利用的癌症漏洞。



























 京公网安备 11010802027423号
京公网安备 11010802027423号