当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tunable Cu(i)-catalyzed site-selective dehydrogenative amination of β,γ-unsaturated hydrazones for divergent synthesis of pyrazol-4-ones and 1,6-dihydropyradazines†
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-09-28 00:00:00 , DOI: 10.1039/c7qo00743d Dong-Fang Jiang 1, 2, 3, 4, 5 , Jie-Yu Hu 1, 2, 3, 4, 5 , Wen-Juan Hao 1, 2, 3, 4, 5 , Shu-Liang Wang 1, 2, 3, 4, 5 , Shu-Jiang Tu 1, 2, 3, 4, 5 , Bo Jiang 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-09-28 00:00:00 , DOI: 10.1039/c7qo00743d Dong-Fang Jiang 1, 2, 3, 4, 5 , Jie-Yu Hu 1, 2, 3, 4, 5 , Wen-Juan Hao 1, 2, 3, 4, 5 , Shu-Liang Wang 1, 2, 3, 4, 5 , Shu-Jiang Tu 1, 2, 3, 4, 5 , Bo Jiang 1, 2, 3, 4, 5
Affiliation
|
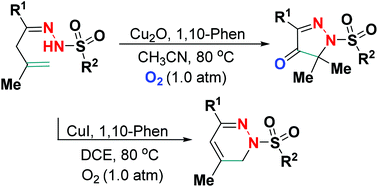
A new tunable Cu(I)-catalyzed site-selective dehydrogenative amination of β,γ-unsaturated hydrazones has been established using readily accessible dioxygen as a green oxidant. The presence of Cu2O/O2 regiospecifically accessed densely functionalized pyrazol-4-ones with one quaternary carbon-amino functionality, in which O2 acted as both a reaction component and an oxidant, whereas the employment of CuI/O2 as a catalytic oxidation system resulted in 1,6-dihydropyradazines with good yields.
中文翻译:

可调谐的Cu(i)催化β,γ-不饱和azo的位点选择性脱氢胺化,用于吡唑-4-酮和1,6-二氢吡嗪的发散合成†
使用容易获得的双氧作为绿色氧化剂,已经建立了一种新的可调谐的Cu(I)催化的β,γ-不饱和azo酮的位点选择性脱氢胺化反应。Cu 2 O / O 2区域特异性地访问具有一个季碳-氨基官能团的致密官能化的吡唑-4-酮,其中O 2既是反应组分又是氧化剂,而使用CuI / O 2作为氧化剂。催化氧化反应制得的1,6-二氢吡嗪类化合物收率很高。
更新日期:2017-09-28
中文翻译:

可调谐的Cu(i)催化β,γ-不饱和azo的位点选择性脱氢胺化,用于吡唑-4-酮和1,6-二氢吡嗪的发散合成†
使用容易获得的双氧作为绿色氧化剂,已经建立了一种新的可调谐的Cu(I)催化的β,γ-不饱和azo酮的位点选择性脱氢胺化反应。Cu 2 O / O 2区域特异性地访问具有一个季碳-氨基官能团的致密官能化的吡唑-4-酮,其中O 2既是反应组分又是氧化剂,而使用CuI / O 2作为氧化剂。催化氧化反应制得的1,6-二氢吡嗪类化合物收率很高。



























 京公网安备 11010802027423号
京公网安备 11010802027423号