当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Conversion of a resistant pollutant, phenol, into green fuels by gasification using supercritical water compressed up to 1000 bar
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2017-09-05 00:00:00 , DOI: 10.1039/c7re00081b Nicolas Martin-Sanchez 1, 2, 3, 4, 5 , M. Jesus Sanchez-Montero 1, 2, 3, 4, 5 , Carmen Izquierdo 1, 2, 3, 4, 5 , Francisco Salvador 1, 2, 3, 4, 5
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2017-09-05 00:00:00 , DOI: 10.1039/c7re00081b Nicolas Martin-Sanchez 1, 2, 3, 4, 5 , M. Jesus Sanchez-Montero 1, 2, 3, 4, 5 , Carmen Izquierdo 1, 2, 3, 4, 5 , Francisco Salvador 1, 2, 3, 4, 5
Affiliation
|
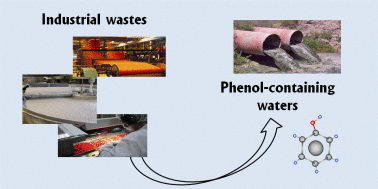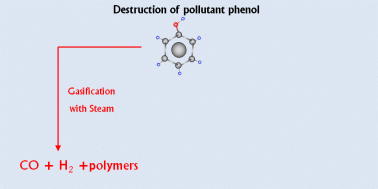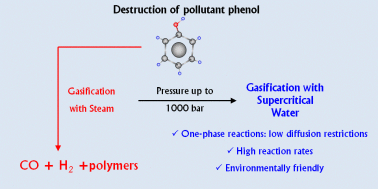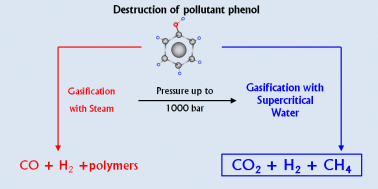
For green sustainable chemistry, it is crucial to investigate the destruction of such a common pollutant as phenol. This study reports the gasification of phenol with steam and supercritical water (SCW) and shows that gasification under high-pressure SCW is a method that destroys and efficiently converts phenol into valuable products. To the best of our knowledge, the widest pressure range ever investigated in this field is utilized, i.e., from atmospheric pressure steam to SCW at 1000 bar. The high temperature used (700 °C) leads to fast degradation of phenol under all the conditions studied but the amount of phenol gasified does strongly depend on pressure. During gasification, polymeric compounds such as naphthalene and phenanthrene are generated. They play a key role in the proposed degradation–gasification mechanism since they are difficult to degrade and can lead to the formation of char. High-pressure SCW can more efficiently degrade and gasify such polymeric compounds compared to steam. Then, the supercritical fluid leads to the conversion of a greater amount of phenol into gas than steam: 68% of the pollutant is gasified at 750 bar and 700 °C after 16.5 min with no generation of pollutant by-products, except CO2. Furthermore, the gaseous stream contains the valuable green gases H2 and CH4. The use of highly compressed SCW implies not only the production of more gases but also the enrichment of the gas mixture in H2 and CH4. H2 and CH4 concentrations up to 35 and 30%, respectively, are obtained at 1000 bar.
中文翻译:

通过使用压缩至1000 bar的超临界水进行气化,将抗性污染物苯酚转化为绿色燃料
对于绿色的可持续化学,至关重要的是研究销毁常见污染物如苯酚的情况。这项研究报告了苯酚与蒸汽和超临界水(SCW)的气化,并表明在高压SCW下气化是一种破坏并有效地将苯酚转化为有价值的产品的方法。据我们所知,利用了该领域有史以来最广泛的压力范围,即,从大气压蒸汽到1000 bar的SCW。在所有研究的条件下,所用的高温(700°C)导致苯酚快速降解,但是气化的苯酚的量确实强烈取决于压力。在气化过程中,生成聚合化合物,例如萘和菲。它们在拟议的降解-气化机制中起着关键作用,因为它们难以降解并且会导致形成焦炭。与蒸汽相比,高压SCW可以更有效地降解和气化此类聚合化合物。然后,与蒸汽相比,超临界流体导致更多的苯酚转化为气体:68%的污染物在16.5分钟后于750 bar和700°C的温度下被气化,除了CO 2以外没有任何污染物副产物的产生。。此外,气流包含有价值的绿色气体H 2和CH 4。使用高度压缩的SCW不仅意味着会产生更多的气体,而且还意味着H 2和CH 4中气体混合物的富集。在1000 bar下获得的H 2和CH 4浓度分别高达35%和30%。
更新日期:2017-09-22
中文翻译:

通过使用压缩至1000 bar的超临界水进行气化,将抗性污染物苯酚转化为绿色燃料
对于绿色的可持续化学,至关重要的是研究销毁常见污染物如苯酚的情况。这项研究报告了苯酚与蒸汽和超临界水(SCW)的气化,并表明在高压SCW下气化是一种破坏并有效地将苯酚转化为有价值的产品的方法。据我们所知,利用了该领域有史以来最广泛的压力范围,即,从大气压蒸汽到1000 bar的SCW。在所有研究的条件下,所用的高温(700°C)导致苯酚快速降解,但是气化的苯酚的量确实强烈取决于压力。在气化过程中,生成聚合化合物,例如萘和菲。它们在拟议的降解-气化机制中起着关键作用,因为它们难以降解并且会导致形成焦炭。与蒸汽相比,高压SCW可以更有效地降解和气化此类聚合化合物。然后,与蒸汽相比,超临界流体导致更多的苯酚转化为气体:68%的污染物在16.5分钟后于750 bar和700°C的温度下被气化,除了CO 2以外没有任何污染物副产物的产生。。此外,气流包含有价值的绿色气体H 2和CH 4。使用高度压缩的SCW不仅意味着会产生更多的气体,而且还意味着H 2和CH 4中气体混合物的富集。在1000 bar下获得的H 2和CH 4浓度分别高达35%和30%。



























 京公网安备 11010802027423号
京公网安备 11010802027423号