当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Biredox ionic liquids: new opportunities toward high performance supercapacitors
Faraday Discussions ( IF 3.4 ) Pub Date : 2017-06-29 00:00:00 , DOI: 10.1039/c7fd00174f C. Bodin 1, 2, 3, 4, 5 , E. Mourad 1, 2, 3, 4, 5 , D. Zigah 5, 6, 7, 8, 9 , S. Le Vot 1, 2, 3, 4, 5 , S. A. Freunberger 10, 11, 12, 13 , F. Favier 1, 2, 3, 4, 5 , O. Fontaine 1, 2, 3, 4, 5
Faraday Discussions ( IF 3.4 ) Pub Date : 2017-06-29 00:00:00 , DOI: 10.1039/c7fd00174f C. Bodin 1, 2, 3, 4, 5 , E. Mourad 1, 2, 3, 4, 5 , D. Zigah 5, 6, 7, 8, 9 , S. Le Vot 1, 2, 3, 4, 5 , S. A. Freunberger 10, 11, 12, 13 , F. Favier 1, 2, 3, 4, 5 , O. Fontaine 1, 2, 3, 4, 5
Affiliation
|
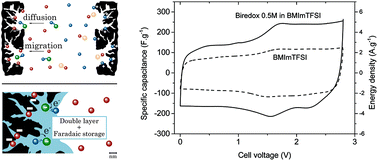
Nowadays commercial supercapacitors are based on purely capacitive storage at the porous carbons that are used for the electrodes. However, the limits that capacitive storage imposes on energy density calls to investigate new materials to improve the capacitance of the device. This new type of electrodes (e.g., RuO2, MnO2…) involves pseudo-capacitive faradaic redox processes with the solid material. Ion exchange with solid materials is, however, much slower than the adsorption process in capacitive storage and inevitably leads to significant loss of power. Faradaic process in the liquid state, in contrast can be similarly fast as capacitive processes due to the fast ion transport. Designing new devices with liquid like dynamics and improved specific capacitance is challenging. We present a new approach to increase the specific capacitance using biredox ionic liquids, where redox moieties are tethered to the electrolyte ions, allowing high redox concentrations and significant pseudo-capacitive storage in the liquid state. Anions and cations are functionalized with anthraquinone (AQ) and 2,2,6,6-tetramethylpiperidinyl-1-oxyl (TEMPO) moieties, respectively. Glassy carbon, carbon-onion, and commercial activated carbon electrodes that exhibit different double layer structures and thus different diffusion dynamics were used to simultaneously study the electrochemical response of biredox ionic liquids at the positive and negative electrode.
中文翻译:

Biredox离子液体:高性能超级电容器的新机遇
如今,商用超级电容器基于在用于电极的多孔碳上的纯电容性存储。但是,电容存储对能量密度的限制要求研究新材料以改善器件的电容。这种新型的电极(例如RuO 2,MnO 2…)涉及使用固态材料的伪电容式法拉第氧化还原过程。但是,与固态材料进行离子交换比电容性存储中的吸附过程要慢得多,并且不可避免地会导致功率的显着损失。相反,由于离子的快速传输,液态的法拉第过程可以与电容过程类似地快速。设计具有动态性和改善的比电容等液体的新设备具有挑战性。我们提出了一种使用双氧化还原离子液体增加比电容的新方法,其中氧化还原部分被束缚到电解质离子上,从而允许高氧化还原浓度和液态下的大量伪电容存储。阴离子和阳离子分别被蒽醌(AQ)和2,2,6,6-四甲基哌啶基-1-氧基(TEMPO)部分官能化。
更新日期:2017-12-15
中文翻译:

Biredox离子液体:高性能超级电容器的新机遇
如今,商用超级电容器基于在用于电极的多孔碳上的纯电容性存储。但是,电容存储对能量密度的限制要求研究新材料以改善器件的电容。这种新型的电极(例如RuO 2,MnO 2…)涉及使用固态材料的伪电容式法拉第氧化还原过程。但是,与固态材料进行离子交换比电容性存储中的吸附过程要慢得多,并且不可避免地会导致功率的显着损失。相反,由于离子的快速传输,液态的法拉第过程可以与电容过程类似地快速。设计具有动态性和改善的比电容等液体的新设备具有挑战性。我们提出了一种使用双氧化还原离子液体增加比电容的新方法,其中氧化还原部分被束缚到电解质离子上,从而允许高氧化还原浓度和液态下的大量伪电容存储。阴离子和阳离子分别被蒽醌(AQ)和2,2,6,6-四甲基哌啶基-1-氧基(TEMPO)部分官能化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号