当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lucky Switcheroo: Dramatic Potency and Selectivity Improvement of Imidazoline Inhibitors of Human Carbonic Anhydrase VII
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00300 Stanislav Kalinin 1 , Stanislav Kopylov 1 , Tiziano Tuccinardi 2 , Alexander Sapegin 1 , Dmitry Dar’in 1 , Andrea Angeli 3 , Claudiu T. Supuran 3 , Mikhail Krasavin 1
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00300 Stanislav Kalinin 1 , Stanislav Kopylov 1 , Tiziano Tuccinardi 2 , Alexander Sapegin 1 , Dmitry Dar’in 1 , Andrea Angeli 3 , Claudiu T. Supuran 3 , Mikhail Krasavin 1
Affiliation
|
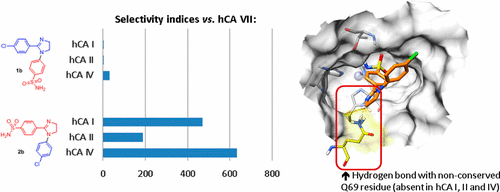
A substantial improvement of potency and selectivity of imidazoline-based inhibitors of hCA VII (a promising target for the treatment of seizures and neuropathic pain) was achieved by simply switching the position of the benzenesulfonamide moiety from N1 (as in the earlier reported series) to C2. Selectivity indices vs the off-target isoforms (hCA I, I, I and IV) greater than 100 were reached, which is exceedingly rare for hCA VII inhibitors. The drastic profile improvement of the new series has been rationalized by an additional hydrogen bonding with the nonconserved Q69 residue in the active site of hCA VII (absent in the other three isoforms studied), which also results in a favorable accommodation of the inhibitor’s lipophilic periphery in the nearby hydrophobic pocket. The robustness of the docking simulations was tested and confirmed by molecular dynamics simulations.
中文翻译:

幸运的Switcheroo:人类碳酸酐酶VII的咪唑啉抑制剂的显着效力和选择性改善
通过简单地将苯磺酰胺部分的位置从N 1切换(如先前报道的系列),可以大大提高基于咪唑啉的hCA VII抑制剂(治疗癫痫和神经性疼痛的有希望的靶点)的效能和选择性。至C 2。相对于脱靶同工型(hCA I,I,I和IV)的选择性指数大于100,这对于hCA VII抑制剂而言极为罕见。通过与hCA VII活性位点中的非保守Q69残基进行额外的氢键结合(在研究的其他三种同工型中不存在),已合理地解释了新系列的显着改善,这也为抑制剂的亲脂性外周提供了良好的适应性在附近的疏水袋中。对接模拟的鲁棒性已通过分子动力学模拟进行了测试和确认。
更新日期:2017-09-20
中文翻译:

幸运的Switcheroo:人类碳酸酐酶VII的咪唑啉抑制剂的显着效力和选择性改善
通过简单地将苯磺酰胺部分的位置从N 1切换(如先前报道的系列),可以大大提高基于咪唑啉的hCA VII抑制剂(治疗癫痫和神经性疼痛的有希望的靶点)的效能和选择性。至C 2。相对于脱靶同工型(hCA I,I,I和IV)的选择性指数大于100,这对于hCA VII抑制剂而言极为罕见。通过与hCA VII活性位点中的非保守Q69残基进行额外的氢键结合(在研究的其他三种同工型中不存在),已合理地解释了新系列的显着改善,这也为抑制剂的亲脂性外周提供了良好的适应性在附近的疏水袋中。对接模拟的鲁棒性已通过分子动力学模拟进行了测试和确认。



























 京公网安备 11010802027423号
京公网安备 11010802027423号