当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficient Thermal Reactions of Sulfur Dioxide on Ice Surfaces at Low Temperature: A Combined Experimental and Theoretical Study
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00064 Jaehyeock Bang 1 , Mahbubul Alam Shoaib 2 , Cheol Ho Choi 2 , Heon Kang 1
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00064 Jaehyeock Bang 1 , Mahbubul Alam Shoaib 2 , Cheol Ho Choi 2 , Heon Kang 1
Affiliation
|
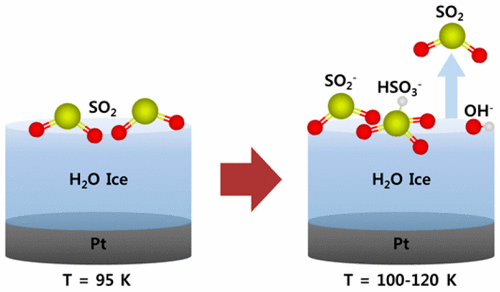
The interaction of sulfur dioxide (SO2) gas with a crystalline ice surface at low temperature was studied by analyzing the surface species with low energy sputtering (LES) and reactive ion scattering methods and the desorbing gases with temperature-programmed desorption mass spectrometry. The study gives direct evidence for the occurrence of efficient hydrolysis of SO2 with low energy barriers on the ice surface. Adsorbed SO2 molecules react with the ice surface at temperatures above ∼90 K to form anionic molecular species, which can be detected by OH–, SO2–, and HSO3– emission signals in the LES experiments. Heating the sample above ∼120 K causes the desorption of SO2 gas from the surface-bound hydrolysis products. As a result, the hydrolysis of SO2 on an ice surface is most efficient at 100–120 K. The surface products formed at these temperatures correspond to metastable states, which are kinetically isolated on the cold surface. Quantum mechanical calculations of a model ice system suggest plausible mechanistic pathways for how physisorbed SO2 is transformed into chemisorbed HSO3– species. HSO3– is formed either by direct conversion of physisorbed SO2 or through the formation of a stable H2SO3 surface complex, both involving proton transfer on the ice surface with low energy barriers. These findings suggest the possibility that thermal reactions of SO2 occur efficiently on the ice surface of Jovian satellites even without bombardment by high-energy radiation.
中文翻译:

二氧化硫在低温下在冰面上的有效热反应:组合的实验和理论研究
通过用低能溅射(LES)和反应性离子散射法分析表面物质,并利用程序升温脱附质谱法分析脱附气体,研究了低温下二氧化硫(SO 2)气体与结晶冰表面的相互作用。该研究为冰表面低能垒的SO 2有效水解提供了直接证据。吸附SO 2个分子与冰表面反应在温度约90ķ以上以形成阴离子性分子物质,其可被OH检测-,SO 2 - ,和HSO 3 -LES实验中的发射信号。将样品加热到约120 K以上会导致SO 2气体从表面结合的水解产物中解吸。结果,冰表面上的SO 2水解在100–120 K时最有效。在这些温度下形成的表面产物对应于亚稳态,该状态在动力学上被隔离在冷表面上。一个模型冰系统的量子力学的计算表明对于如何SO物理吸附似是而非的机制途径2被转化成化学吸附HSO 3 -物种。HSO 3 –是通过直接吸收物理吸附的SO 2或通过形成稳定的H 2 SO形成的3表面复合物,均涉及质子在冰表面上的传递,具有较低的能垒。这些发现表明,即使没有受到高能辐射的轰击,SO 2的热反应仍可能在木星卫星的冰面上有效地发生。
更新日期:2017-09-20
中文翻译:

二氧化硫在低温下在冰面上的有效热反应:组合的实验和理论研究
通过用低能溅射(LES)和反应性离子散射法分析表面物质,并利用程序升温脱附质谱法分析脱附气体,研究了低温下二氧化硫(SO 2)气体与结晶冰表面的相互作用。该研究为冰表面低能垒的SO 2有效水解提供了直接证据。吸附SO 2个分子与冰表面反应在温度约90ķ以上以形成阴离子性分子物质,其可被OH检测-,SO 2 - ,和HSO 3 -LES实验中的发射信号。将样品加热到约120 K以上会导致SO 2气体从表面结合的水解产物中解吸。结果,冰表面上的SO 2水解在100–120 K时最有效。在这些温度下形成的表面产物对应于亚稳态,该状态在动力学上被隔离在冷表面上。一个模型冰系统的量子力学的计算表明对于如何SO物理吸附似是而非的机制途径2被转化成化学吸附HSO 3 -物种。HSO 3 –是通过直接吸收物理吸附的SO 2或通过形成稳定的H 2 SO形成的3表面复合物,均涉及质子在冰表面上的传递,具有较低的能垒。这些发现表明,即使没有受到高能辐射的轰击,SO 2的热反应仍可能在木星卫星的冰面上有效地发生。


























 京公网安备 11010802027423号
京公网安备 11010802027423号