当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rates of CO2 Mineralization in Geological Carbon Storage
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2017-08-28 00:00:00 , DOI: 10.1021/acs.accounts.7b00334 Shuo Zhang 1, 2 , Donald J. DePaolo 1, 3
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2017-08-28 00:00:00 , DOI: 10.1021/acs.accounts.7b00334 Shuo Zhang 1, 2 , Donald J. DePaolo 1, 3
Affiliation
|
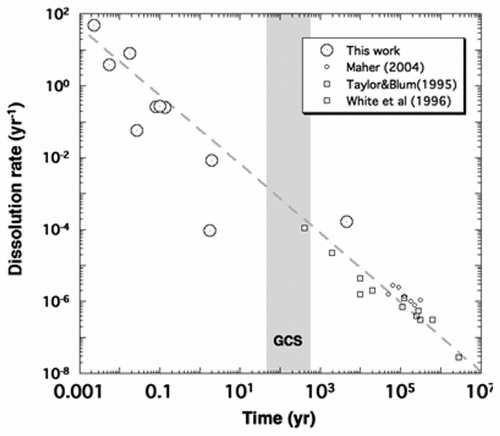
Geologic carbon storage (GCS) involves capture and purification of CO2 at industrial emission sources, compression into a supercritical state, and subsequent injection into geologic formations. This process reverses the flow of carbon to the atmosphere with the intention of returning the carbon to long-term geologic storage. Models suggest that most of the injected CO2 will be “trapped” in the subsurface by physical means, but the most risk-free and permanent form of carbon storage is as carbonate minerals (Ca,Mg,Fe)CO3. The transformation of CO2 to carbonate minerals requires supply of the necessary divalent cations by dissolution of silicate minerals. Available data suggest that rates of transformation are highly uncertain and difficult to predict by standard approaches. Here we show that the chemical kinetic observations and experimental results, when they can be reduced to a single cation-release time scale that describes the fractional rate at which cations are released to solution by mineral dissolution, show sufficiently systematic behavior as a function of pH, fluid flow rate, and time that the rates of mineralization can be estimated with reasonable certainty.
中文翻译:

地质碳储量中CO 2矿化速率
地质碳储存(GCS)涉及在工业排放源处捕获和纯化CO 2,压缩成超临界状态,然后注入地质构造中。此过程使碳向大气的流动方向反向,目的是使碳返回长期的地质存储。模型表明,大多数注入的CO 2将通过物理方式“捕获”在地下,但是最无风险和永久性的碳存储形式是碳酸盐矿物(Ca,Mg,Fe)CO 3。CO 2的转化碳酸盐矿物的分解需要通过溶解硅酸盐矿物来提供必要的二价阳离子。现有数据表明,转化率高度不确定,很难用标准方法预测。在这里,我们表明,当化学动力学观察结果和实验结果可以减少为一个单一的阳离子释放时间尺度时,该时间尺度描述了通过矿物溶解将阳离子释放到溶液中的分数速率时,它们显示出足够的系统行为,该行为是pH的函数,流体流速和时间,可以合理地确定矿化速率。
更新日期:2017-08-28
中文翻译:

地质碳储量中CO 2矿化速率
地质碳储存(GCS)涉及在工业排放源处捕获和纯化CO 2,压缩成超临界状态,然后注入地质构造中。此过程使碳向大气的流动方向反向,目的是使碳返回长期的地质存储。模型表明,大多数注入的CO 2将通过物理方式“捕获”在地下,但是最无风险和永久性的碳存储形式是碳酸盐矿物(Ca,Mg,Fe)CO 3。CO 2的转化碳酸盐矿物的分解需要通过溶解硅酸盐矿物来提供必要的二价阳离子。现有数据表明,转化率高度不确定,很难用标准方法预测。在这里,我们表明,当化学动力学观察结果和实验结果可以减少为一个单一的阳离子释放时间尺度时,该时间尺度描述了通过矿物溶解将阳离子释放到溶液中的分数速率时,它们显示出足够的系统行为,该行为是pH的函数,流体流速和时间,可以合理地确定矿化速率。



























 京公网安备 11010802027423号
京公网安备 11010802027423号