当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of Pharmaceutically Acceptable Crystalline Forms of Drug Substances via Solid-State Solvent Exchange
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2017-08-25 00:00:00 , DOI: 10.1021/acs.oprd.7b00114 Srividya Ramakrishnan 1 , Sesha Reddy Yarraguntla 1 , Subba Reddy Peddireddy 1 , Sundara Lakshmi Kanniah 1 , Vamsi Krishna Mudapaka 1 , Lalita Kanwar Shekhawat 1 , Sudarshan Mahapatra 1 , Amjad Basha Mohammad 1 , Peddy Vishweshwar 1 , Peter W. Stephens 2
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2017-08-25 00:00:00 , DOI: 10.1021/acs.oprd.7b00114 Srividya Ramakrishnan 1 , Sesha Reddy Yarraguntla 1 , Subba Reddy Peddireddy 1 , Sundara Lakshmi Kanniah 1 , Vamsi Krishna Mudapaka 1 , Lalita Kanwar Shekhawat 1 , Sudarshan Mahapatra 1 , Amjad Basha Mohammad 1 , Peddy Vishweshwar 1 , Peter W. Stephens 2
Affiliation
|
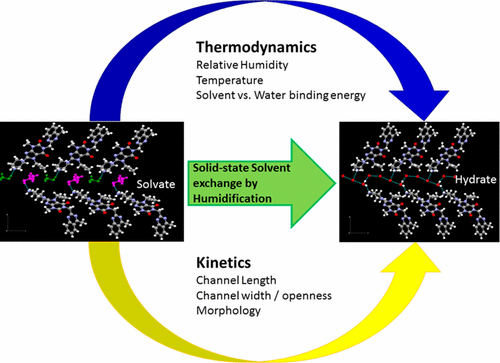
In the pharmaceutical industry, solid form screening plays an important role to discover forms that exhibit desired physicochemical properties for drug product development. This work describes an approach to meet this objective by the transformation of undesirable solvates to hydrates or cosolvates with water via solid-state solvent exchange. Case studies of two drug substances, imatinib mesylate and linagliptin, are discussed, where linaglipitin methanol/ethanol solvate was converted to an iso-structural hydrate and, similarly, imatinib mesylate methanol–water cosolvate was converted to a predominantly water-containing cosolvate. Through quality by design based optimization, temperature and relative humidity were identified as critical process parameters that impacted the rate of solvent exchange during humidification. In addition, crystallization parameters that impacted the crystal size were found to play a key role in determining the extent of solvent exchange. This unexpected effect of crystal size was investigated through single crystal structure elucidation and molecular modeling, which showed the solvent to be residing in channels oriented along the length of the crystal. The dimensions of these channels determined the ease of solvent exchange by controlling the rate and extent of diffusion of solvent molecules. With these case studies, this paper provides insight on robust process development for solid-state solvent exchange with an in-depth understanding of molecular level phenomena and critical process parameters.
中文翻译:

通过固态溶剂交换开发可药用晶体形式的药物
在制药工业中,固体形式筛选在发现表现出对药物产品开发具有所需理化特性的形式方面起着重要作用。这项工作描述了一种通过固态溶剂交换将不希望的溶剂化物转化为水合物或与水共溶剂的方法来实现这一目标。讨论了两种药物物质(甲磺酸伊马替尼和利格列汀)的案例研究,其中利那利汀甲醇/乙醇溶剂化物转化为同结构水合物,类似地,甲磺酸伊马替尼甲醇-水共溶剂化物转化为主要为水的共溶剂化物。通过基于设计的优化质量,温度和相对湿度被确定为影响加湿过程中溶剂交换速率的关键工艺参数。此外,发现影响晶体尺寸的结晶参数在确定溶剂交换程度方面起关键作用。通过单晶结构阐明和分子建模研究了晶体大小的这种出乎意料的影响,这表明溶剂存在于沿晶体长度方向的通道中。这些通道的尺寸通过控制溶剂分子的扩散速率和程度决定了溶剂交换的难易程度。通过这些案例研究,本文深入了解分子水平现象和关键工艺参数,从而为固态溶剂交换的稳健工艺开发提供了见识。通过单晶结构阐明和分子建模研究了晶体大小的这种出乎意料的影响,这表明溶剂存在于沿晶体长度方向的通道中。这些通道的尺寸通过控制溶剂分子的扩散速率和程度决定了溶剂交换的难易程度。通过这些案例研究,本文深入了解分子水平现象和关键工艺参数,从而为固态溶剂交换的稳健工艺开发提供了见识。通过单晶结构阐明和分子建模研究了晶体大小的这种出乎意料的影响,这表明溶剂存在于沿晶体长度方向的通道中。这些通道的尺寸通过控制溶剂分子的扩散速率和程度决定了溶剂交换的难易程度。通过这些案例研究,本文深入了解分子水平现象和关键工艺参数,从而为固态溶剂交换的稳健工艺开发提供了见识。这些通道的尺寸通过控制溶剂分子的扩散速率和程度决定了溶剂交换的难易程度。通过这些案例研究,本文深入了解分子水平现象和关键工艺参数,从而为固态溶剂交换的稳健工艺开发提供了见识。这些通道的尺寸通过控制溶剂分子的扩散速率和程度决定了溶剂交换的难易程度。通过这些案例研究,本文深入了解分子水平现象和关键工艺参数,从而为固态溶剂交换的稳健工艺开发提供了见识。
更新日期:2017-08-25
中文翻译:

通过固态溶剂交换开发可药用晶体形式的药物
在制药工业中,固体形式筛选在发现表现出对药物产品开发具有所需理化特性的形式方面起着重要作用。这项工作描述了一种通过固态溶剂交换将不希望的溶剂化物转化为水合物或与水共溶剂的方法来实现这一目标。讨论了两种药物物质(甲磺酸伊马替尼和利格列汀)的案例研究,其中利那利汀甲醇/乙醇溶剂化物转化为同结构水合物,类似地,甲磺酸伊马替尼甲醇-水共溶剂化物转化为主要为水的共溶剂化物。通过基于设计的优化质量,温度和相对湿度被确定为影响加湿过程中溶剂交换速率的关键工艺参数。此外,发现影响晶体尺寸的结晶参数在确定溶剂交换程度方面起关键作用。通过单晶结构阐明和分子建模研究了晶体大小的这种出乎意料的影响,这表明溶剂存在于沿晶体长度方向的通道中。这些通道的尺寸通过控制溶剂分子的扩散速率和程度决定了溶剂交换的难易程度。通过这些案例研究,本文深入了解分子水平现象和关键工艺参数,从而为固态溶剂交换的稳健工艺开发提供了见识。通过单晶结构阐明和分子建模研究了晶体大小的这种出乎意料的影响,这表明溶剂存在于沿晶体长度方向的通道中。这些通道的尺寸通过控制溶剂分子的扩散速率和程度决定了溶剂交换的难易程度。通过这些案例研究,本文深入了解分子水平现象和关键工艺参数,从而为固态溶剂交换的稳健工艺开发提供了见识。通过单晶结构阐明和分子建模研究了晶体大小的这种出乎意料的影响,这表明溶剂存在于沿晶体长度方向的通道中。这些通道的尺寸通过控制溶剂分子的扩散速率和程度决定了溶剂交换的难易程度。通过这些案例研究,本文深入了解分子水平现象和关键工艺参数,从而为固态溶剂交换的稳健工艺开发提供了见识。这些通道的尺寸通过控制溶剂分子的扩散速率和程度决定了溶剂交换的难易程度。通过这些案例研究,本文深入了解分子水平现象和关键工艺参数,从而为固态溶剂交换的稳健工艺开发提供了见识。这些通道的尺寸通过控制溶剂分子的扩散速率和程度决定了溶剂交换的难易程度。通过这些案例研究,本文深入了解分子水平现象和关键工艺参数,从而为固态溶剂交换的稳健工艺开发提供了见识。


























 京公网安备 11010802027423号
京公网安备 11010802027423号