Joule ( IF 39.8 ) Pub Date : 2017-08-09 , DOI: 10.1016/j.joule.2017.07.001 Yu Qiao , Jin Yi , Shichao Wu , Yang Liu , Sixie Yang , Ping He , Haoshen Zhou
|
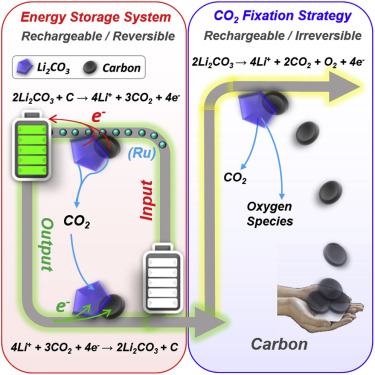
Large energy is required for traditional CO2 fixation, leading to more CO2 emission and additional pollutants. Recently, integrating renewable energy with CO2 fixation has attracted increasing attention as a sustainable strategy. Here, based on a systematic investigation on aprotic Li-CO2 electrochemistry, we first provide an alternative strategy for either CO2 fixation or energy storage. Both strategies share the same CO2 reduction process with the formation of Li2CO3 and carbon. Subsequently, CO2 fixation is achieved through a rechargeable/irreversible oxidation process, during which Li2CO3 is decomposed, while the carbon obtained remains fixed. Moreover, a reversible Li-CO2 battery system has been realized based on co-oxidization of the resulting carbon and Li2CO3 using a Ru catalyst. Consequently, by shedding light on the fundamental reaction mechanism of aprotic Li-CO2 electrochemistry, the proof of concept presented here provides strong theoretical underpinning for developing flexible routes for both CO2 fixation and Li-CO2 energy storage.
中文翻译:

Li-CO 2电化学:CO 2固定和储能的新策略
传统的CO 2固定需要大量的能量,导致更多的CO 2排放和更多的污染物。近来,将可再生能源与CO 2固定结合起来作为一种可持续的策略已引起越来越多的关注。在此,基于对质子惰性Li-CO 2电化学的系统研究,我们首先为CO 2固定或能量存储提供了另一种策略。两种策略在形成Li 2 CO 3和碳时具有相同的CO 2还原过程。随后,通过可充电/不可逆的氧化过程实现CO 2固定,在此过程中,Li 2CO 3分解,同时获得的碳保持固定。此外,基于使用Ru催化剂对所得碳和Li 2 CO 3的共氧化,已经实现了可逆Li-CO 2电池系统。因此,通过阐明非质子性Li-CO 2电化学的基本反应机理,本文提出的概念证明为开发灵活的CO 2固定和Li-CO 2能量存储途径提供了强有力的理论基础。


























 京公网安备 11010802027423号
京公网安备 11010802027423号