当前位置:
X-MOL 学术
›
Nanoscale Horiz.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetic enhancement in high-activity enzyme complexes attached to nanoparticles
Nanoscale Horizons ( IF 9.7 ) Pub Date : 2017-07-25 00:00:00 , DOI: 10.1039/c7nh00052a Anthony P. Malanoski 1, 2, 3, 4, 5 , Joyce C. Breger 1, 2, 3, 4, 5 , Carl W. Brown 1, 2, 3, 4, 5 , Jeffrey R. Deschamps 1, 2, 3, 4, 5 , Kimihiro Susumu 3, 4, 5, 6, 7 , Eunkeu Oh 3, 4, 5, 6, 7 , George P. Anderson 1, 2, 3, 4, 5 , Scott A. Walper 1, 2, 3, 4, 5 , Igor L. Medintz 1, 2, 3, 4, 5
Nanoscale Horizons ( IF 9.7 ) Pub Date : 2017-07-25 00:00:00 , DOI: 10.1039/c7nh00052a Anthony P. Malanoski 1, 2, 3, 4, 5 , Joyce C. Breger 1, 2, 3, 4, 5 , Carl W. Brown 1, 2, 3, 4, 5 , Jeffrey R. Deschamps 1, 2, 3, 4, 5 , Kimihiro Susumu 3, 4, 5, 6, 7 , Eunkeu Oh 3, 4, 5, 6, 7 , George P. Anderson 1, 2, 3, 4, 5 , Scott A. Walper 1, 2, 3, 4, 5 , Igor L. Medintz 1, 2, 3, 4, 5
Affiliation
|
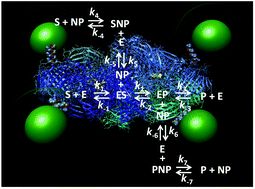
Accumulating studies by many groups have found consistent enhancement in a wide variety of enzyme activities when they are displayed around nanoparticles. However, the underlying mechanism(s) that give rise to this phenomenon are still largely unknown. Herein, we develop a detailed reaction scheme that considers many of the various possible interactions between a substrate and a given enzyme–nanoparticle bioconjugate. The properties and some functional predictions that emanate from the reaction scheme were then tested using a model system where the homotetrameric beta-galactosidase enzyme complex was assembled with luminescent semiconductor nanocrystalline quantum dots displayed around its periphery. This type of assembly occurs as the ∼465 kDa enzyme complex is significantly larger than the 4.2 nm diameter green emitting quantum dots utilized. This unique architecture, in conjunction with the fact that this enzyme functions at or near the diffusion limit, provided a unique opportunity to selectively probe certain aspects of enzyme enhancement when attached to a nanoparticle with minimal potential perturbations to the native enzyme structure. Experimental assays were conducted where both free enzymes and quantum dot-decorated enzymes were compared directly in side-by-side samples and included formats where the kinetic processes were challenged with increasing viscosity and competitive inhibitors. The results strongly suggest that it is possible for there to be significant enhancements in an enzyme's catalytic rate or kcat after attachment to a nanoparticle even when it is apparently diffusion limited without requiring any gross changes to the enzyme's structure. A discussion of how this reaction scheme and model can be applied to other systems is provided.
中文翻译:

附着在纳米颗粒上的高活性酶复合物的动力学增强
许多小组进行的积累研究发现,当它们显示在纳米粒子周围时,它们在各种酶活性中的作用都将持续增强。但是,引起这种现象的潜在机制仍是未知之数。本文中,我们开发了一种详细的反应方案,该方案考虑了底物与给定的酶-纳米颗粒生物结合物之间的各种可能的相互作用。然后使用模型系统测试由反应方案产生的性质和一些功能预测,其中同四聚体β-半乳糖苷酶复合物与在其外围显示的发光半导体纳米晶体量子点组装在一起。这种组装的发生是因为〜465 kDa的酶复合物明显大于所利用的4.2 nm直径的绿色发射量子点。这种独特的结构,结合该酶在扩散极限处或附近起作用的事实,提供了一个独特的机会,可以选择性地探测与纳米颗粒连接的酶增强的某些方面,而对天然酶结构的潜在干扰最小。进行了实验测定,其中直接比较了并排样品中的游离酶和量子点修饰的酶,并包括了动力学过程受到粘度增加和竞争性抑制剂挑战的形式。结果强烈表明,酶的催化速率可能显着提高,或者 提供了一个独特的机会,当它附着到纳米颗粒上时,选择性地探测酶增强的某些方面,而对天然酶结构的潜在干扰最小。进行了实验测定,其中直接比较了并排样品中的游离酶和量子点修饰的酶,并包括了动力学过程受到粘度增加和竞争性抑制剂挑战的形式。结果强烈表明,酶的催化速率可能显着提高,或者 提供了一个独特的机会,当它附着到纳米颗粒上时,选择性地探测酶增强的某些方面,而对天然酶结构的潜在干扰最小。进行了实验测定,其中直接比较了并排样品中的游离酶和量子点修饰的酶,并包括了动力学过程受到粘度增加和竞争性抑制剂挑战的形式。结果强烈表明,酶的催化速率可能显着提高,或者 进行了实验测定,其中直接比较了并排样品中的游离酶和量子点修饰的酶,并包括了动力学过程受到粘度增加和竞争性抑制剂挑战的形式。结果强烈表明,酶的催化速率可能显着提高,或者 进行了实验测定,其中直接比较了并排样品中的游离酶和量子点修饰的酶,并包括了动力学过程受到粘度增加和竞争性抑制剂挑战的形式。结果强烈表明,酶的催化速率可能显着提高,或者附着在纳米粒子上后的k cat,即使它显然受到扩散限制,而无需对酶的结构进行任何大的改变。提供了有关如何将该反应方案和模型应用于其他系统的讨论。
更新日期:2017-08-21
中文翻译:

附着在纳米颗粒上的高活性酶复合物的动力学增强
许多小组进行的积累研究发现,当它们显示在纳米粒子周围时,它们在各种酶活性中的作用都将持续增强。但是,引起这种现象的潜在机制仍是未知之数。本文中,我们开发了一种详细的反应方案,该方案考虑了底物与给定的酶-纳米颗粒生物结合物之间的各种可能的相互作用。然后使用模型系统测试由反应方案产生的性质和一些功能预测,其中同四聚体β-半乳糖苷酶复合物与在其外围显示的发光半导体纳米晶体量子点组装在一起。这种组装的发生是因为〜465 kDa的酶复合物明显大于所利用的4.2 nm直径的绿色发射量子点。这种独特的结构,结合该酶在扩散极限处或附近起作用的事实,提供了一个独特的机会,可以选择性地探测与纳米颗粒连接的酶增强的某些方面,而对天然酶结构的潜在干扰最小。进行了实验测定,其中直接比较了并排样品中的游离酶和量子点修饰的酶,并包括了动力学过程受到粘度增加和竞争性抑制剂挑战的形式。结果强烈表明,酶的催化速率可能显着提高,或者 提供了一个独特的机会,当它附着到纳米颗粒上时,选择性地探测酶增强的某些方面,而对天然酶结构的潜在干扰最小。进行了实验测定,其中直接比较了并排样品中的游离酶和量子点修饰的酶,并包括了动力学过程受到粘度增加和竞争性抑制剂挑战的形式。结果强烈表明,酶的催化速率可能显着提高,或者 提供了一个独特的机会,当它附着到纳米颗粒上时,选择性地探测酶增强的某些方面,而对天然酶结构的潜在干扰最小。进行了实验测定,其中直接比较了并排样品中的游离酶和量子点修饰的酶,并包括了动力学过程受到粘度增加和竞争性抑制剂挑战的形式。结果强烈表明,酶的催化速率可能显着提高,或者 进行了实验测定,其中直接比较了并排样品中的游离酶和量子点修饰的酶,并包括了动力学过程受到粘度增加和竞争性抑制剂挑战的形式。结果强烈表明,酶的催化速率可能显着提高,或者 进行了实验测定,其中直接比较了并排样品中的游离酶和量子点修饰的酶,并包括了动力学过程受到粘度增加和竞争性抑制剂挑战的形式。结果强烈表明,酶的催化速率可能显着提高,或者附着在纳米粒子上后的k cat,即使它显然受到扩散限制,而无需对酶的结构进行任何大的改变。提供了有关如何将该反应方案和模型应用于其他系统的讨论。



























 京公网安备 11010802027423号
京公网安备 11010802027423号