当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural and Functional Survey of Environmental Aminoglycoside Acetyltransferases Reveals Functionality of Resistance Enzymes.
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2017-08-16 , DOI: 10.1021/acsinfecdis.7b00068 Zhiyu Xu 1 , Peter J Stogios 1, 2 , Andrew T Quaile 1 , Kevin J Forsberg 3 , Sanket Patel 3, 4 , Tatiana Skarina 1, 2 , Scott Houliston 5 , Cheryl Arrowsmith 5 , Gautam Dantas 3, 4, 6, 7 , Alexei Savchenko 1, 2, 8
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2017-08-16 , DOI: 10.1021/acsinfecdis.7b00068 Zhiyu Xu 1 , Peter J Stogios 1, 2 , Andrew T Quaile 1 , Kevin J Forsberg 3 , Sanket Patel 3, 4 , Tatiana Skarina 1, 2 , Scott Houliston 5 , Cheryl Arrowsmith 5 , Gautam Dantas 3, 4, 6, 7 , Alexei Savchenko 1, 2, 8
Affiliation
|
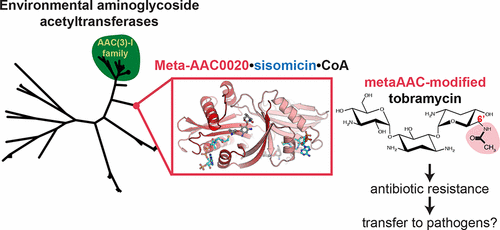
Aminoglycoside N-acetyltransferases (AACs) confer resistance against the clinical use of aminoglycoside antibiotics. The origin of AACs can be traced to environmental microbial species representing a vast reservoir for new and emerging resistance enzymes, which are currently undercharacterized. Here, we performed detailed structural characterization and functional analyses of four metagenomic AAC (meta-AACs) enzymes recently identified in a survey of agricultural and grassland soil microbiomes ( Forsberg et al. Nature 2014 , 509 , 612 ). These enzymes are new members of the Gcn5-Related-N-Acetyltransferase superfamily and confer resistance to the aminoglycosides gentamicin C, sisomicin, and tobramycin. Moreover, the meta-AAC0020 enzyme demonstrated activity comparable with an AAC(3)-I enzyme that serves as a model AAC enzyme identified in a clinical bacterial isolate. The crystal structure of meta-AAC0020 in complex with sisomicin confirmed an unexpected AAC(6') regiospecificity of this enzyme and revealed a drug binding mechanism distinct from previously characterized AAC(6') enzymes. Together, our data highlights the presence of highly active antibiotic-modifying enzymes in the environmental microbiome and reveals unexpected diversity in substrate specificity. These observations of additional AAC enzymes must be considered in the search for novel aminoglycosides less prone to resistance.
中文翻译:

环境氨基糖苷乙酰基转移酶的结构和功能研究揭示了抗性酶的功能。
氨基糖苷N-乙酰基转移酶(AAC)赋予了对氨基糖苷抗生素临床使用的抵抗力。AAC的起源可以追溯到环境微生物物种,这些微生物代表了新的和新兴的抗性酶的巨大储藏库,目前这些酶的特征尚不足。在这里,我们对农业和草原土壤微生物群落的调查中最近鉴定出的四种宏基因组AAC(meta-AAC)酶进行了详细的结构表征和功能分析(Forsberg et al。Nature 2014,509,612)。这些酶是Gcn5-Related-N-乙酰基转移酶超家族的新成员,对氨基糖苷庆大霉素C,西索霉素和妥布霉素具有抗性。而且,meta-AAC0020酶的活性与AAC(3)-I酶相当,后者可作为临床细菌分离物中鉴定的模型AAC酶。meta-AAC0020与西索霉素的复合物的晶体结构证实了该酶具有出乎意料的AAC(6')区域特异性,并揭示了与先前表征的AAC(6')酶不同的药物结合机制。总之,我们的数据突出了环境微生物组中高活性抗生素修饰酶的存在,并揭示了底物特异性方面出乎意料的多样性。在寻找不易产生耐药性的新型氨基糖苷时,必须考虑其他AAC酶的这些观察结果。)这种酶的区域特异性,并揭示了与以前表征的AAC(6')酶不同的药物结合机制。总之,我们的数据突出了环境微生物组中高活性抗生素修饰酶的存在,并揭示了底物特异性方面出乎意料的多样性。在寻找不易产生耐药性的新型氨基糖苷时,必须考虑其他AAC酶的这些观察结果。)这种酶的区域特异性,并揭示了与以前表征的AAC(6')酶不同的药物结合机制。总之,我们的数据突出了环境微生物组中高活性抗生素修饰酶的存在,并揭示了底物特异性方面出乎意料的多样性。在寻找不易产生耐药性的新型氨基糖苷时,必须考虑其他AAC酶的这些观察结果。
更新日期:2017-08-16
中文翻译:

环境氨基糖苷乙酰基转移酶的结构和功能研究揭示了抗性酶的功能。
氨基糖苷N-乙酰基转移酶(AAC)赋予了对氨基糖苷抗生素临床使用的抵抗力。AAC的起源可以追溯到环境微生物物种,这些微生物代表了新的和新兴的抗性酶的巨大储藏库,目前这些酶的特征尚不足。在这里,我们对农业和草原土壤微生物群落的调查中最近鉴定出的四种宏基因组AAC(meta-AAC)酶进行了详细的结构表征和功能分析(Forsberg et al。Nature 2014,509,612)。这些酶是Gcn5-Related-N-乙酰基转移酶超家族的新成员,对氨基糖苷庆大霉素C,西索霉素和妥布霉素具有抗性。而且,meta-AAC0020酶的活性与AAC(3)-I酶相当,后者可作为临床细菌分离物中鉴定的模型AAC酶。meta-AAC0020与西索霉素的复合物的晶体结构证实了该酶具有出乎意料的AAC(6')区域特异性,并揭示了与先前表征的AAC(6')酶不同的药物结合机制。总之,我们的数据突出了环境微生物组中高活性抗生素修饰酶的存在,并揭示了底物特异性方面出乎意料的多样性。在寻找不易产生耐药性的新型氨基糖苷时,必须考虑其他AAC酶的这些观察结果。)这种酶的区域特异性,并揭示了与以前表征的AAC(6')酶不同的药物结合机制。总之,我们的数据突出了环境微生物组中高活性抗生素修饰酶的存在,并揭示了底物特异性方面出乎意料的多样性。在寻找不易产生耐药性的新型氨基糖苷时,必须考虑其他AAC酶的这些观察结果。)这种酶的区域特异性,并揭示了与以前表征的AAC(6')酶不同的药物结合机制。总之,我们的数据突出了环境微生物组中高活性抗生素修饰酶的存在,并揭示了底物特异性方面出乎意料的多样性。在寻找不易产生耐药性的新型氨基糖苷时,必须考虑其他AAC酶的这些观察结果。


























 京公网安备 11010802027423号
京公网安备 11010802027423号