当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydration Is the Key for Gold Transport in CO2–HCl–H2O Vapor
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2017-08-08 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00020 Yuan Mei 1, 2 , Weihua Liu 1 , Joël Brugger 2 , Art A. Migdisov 3 , A. E. Williams-Jones 4
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2017-08-08 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00020 Yuan Mei 1, 2 , Weihua Liu 1 , Joël Brugger 2 , Art A. Migdisov 3 , A. E. Williams-Jones 4
Affiliation
|
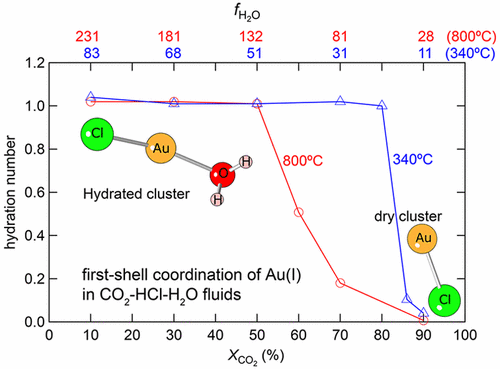
Carbon dioxide (CO2) is a major component of volcanic gases and ore-forming hydrothermal fluids. However, CO2 has contrasting effects on the speciation of different metal complexes and ore mineral solubility, but a molecular understanding of its effects is lacking. To address this deficiency, we conducted ab initio molecular dynamics (MD) simulations of the behavior of AuCl(aq) in the CO2–H2O system at 340 °C and 118–152 bar and 800 °C and 265–291 bar for CO2 mole fractions (XCO2) of 0.1–0.9. The MD simulations indicate that the linear [H2O–Au–Cl]0 structure of gold chloride is not affected by CO2 at XCO2 up to 0.8 at 340 °C and XCO2 up to 0.5 at 800 °C, whereas the “dry” [AuCl]0 species predominates at XCO2 > 0.8 at 340 °C and XCO2 > 0.5 at 800 °C. The number of water molecules hydrating the AuCl(aq) complex decreases systematically with an increasing CO2 mole fraction and increasing temperature. Results of Au solubility experiments at 340 °C in CO2–H2O solutions show that the addition of CO2 does not enhance Au solubility. We conclude that hydrated chloride species with linear geometry are the main means for transporting gold in CO2–H2O–HCl fluids and that Au solubility decreases in CO2-bearing hydrothermal fluids as a result of the decrease in hydration of the Au complexes. This contrasts with the behavior of divalent transition metals (e.g., Fe, Co, Ni, and Zn). We propose that the different solubility behaviors of Au and base metals are due to the changes in translational entropy as a result of the changes in coordination geometry (and associated hydration) of the complexes with increasing XCO2. The first-shell coordination of Au(I) complexes remains constant over wide ranges of XCO2, whereas first-row divalent transition metal complexes undergo entropy-driven geometric changes with a decreasing water activity.
中文翻译:

水合是CO 2 -HCl-H 2 O蒸气中金迁移的关键
二氧化碳(CO 2)是火山气体和成矿热液的主要成分。然而,CO 2对不同金属络合物的形态和矿石矿物溶解度具有相反的影响,但是缺乏对其影响的分子理解。为了解决这一缺陷,我们对340°C,118–152 bar和800°C和265–291 bar的CO 2 –H 2 O系统中的AuCl (aq)行为进行了从头算分子动力学(MD)模拟。对于CO 2摩尔分数(X CO 2)为0.1–0.9。MD模拟表明线性[H 2 O–Au–Cl] 0氯化金结构不受CO 2在X CO 2高达0.8在340℃下和X CO 2达0.5在800℃下,而“干燥” [AUCL] 0在物种占优势X CO 2 > 0.8在340°C时,X CO 2 > 0.5在800°C时。随着CO 2摩尔分数的增加和温度的升高,水化AuCl (aq)络合物的水分子数量系统地减少。在340°C的CO 2 -H 2 O溶液中的Au溶解度实验结果表明,添加了CO 2不会增强金的溶解度。我们得出结论,具有线性几何形状的水合氯化物是在CO 2 -H 2 O-HCl流体中运输金的主要手段,并且由于含Au 2配合物的水合作用减少,含CO 2的热液中的Au溶解度降低。这与二价过渡金属(例如,Fe,Co,Ni和Zn)的行为形成对比。我们提出金和贱金属的不同溶解度行为是由于随着X CO 2的增加,配合物的配位几何形状(和相关的水合作用)的变化,导致了平移熵的变化。Au(I)配合物的第一层配位在很宽的范围内保持恒定X CO 2,而第一行的二价过渡金属络合物则经历了熵驱动的几何变化,且水活度降低。
更新日期:2017-08-09
中文翻译:

水合是CO 2 -HCl-H 2 O蒸气中金迁移的关键
二氧化碳(CO 2)是火山气体和成矿热液的主要成分。然而,CO 2对不同金属络合物的形态和矿石矿物溶解度具有相反的影响,但是缺乏对其影响的分子理解。为了解决这一缺陷,我们对340°C,118–152 bar和800°C和265–291 bar的CO 2 –H 2 O系统中的AuCl (aq)行为进行了从头算分子动力学(MD)模拟。对于CO 2摩尔分数(X CO 2)为0.1–0.9。MD模拟表明线性[H 2 O–Au–Cl] 0氯化金结构不受CO 2在X CO 2高达0.8在340℃下和X CO 2达0.5在800℃下,而“干燥” [AUCL] 0在物种占优势X CO 2 > 0.8在340°C时,X CO 2 > 0.5在800°C时。随着CO 2摩尔分数的增加和温度的升高,水化AuCl (aq)络合物的水分子数量系统地减少。在340°C的CO 2 -H 2 O溶液中的Au溶解度实验结果表明,添加了CO 2不会增强金的溶解度。我们得出结论,具有线性几何形状的水合氯化物是在CO 2 -H 2 O-HCl流体中运输金的主要手段,并且由于含Au 2配合物的水合作用减少,含CO 2的热液中的Au溶解度降低。这与二价过渡金属(例如,Fe,Co,Ni和Zn)的行为形成对比。我们提出金和贱金属的不同溶解度行为是由于随着X CO 2的增加,配合物的配位几何形状(和相关的水合作用)的变化,导致了平移熵的变化。Au(I)配合物的第一层配位在很宽的范围内保持恒定X CO 2,而第一行的二价过渡金属络合物则经历了熵驱动的几何变化,且水活度降低。



























 京公网安备 11010802027423号
京公网安备 11010802027423号