当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Basis for Xenosiderophore Utilization by the Human Pathogen Staphylococcus aureus
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2017-05-30 00:00:00 , DOI: 10.1021/acsinfecdis.7b00036 Nathaniel P. Endicott 1 , Eries Lee 1 , Timothy A. Wencewicz 1
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2017-05-30 00:00:00 , DOI: 10.1021/acsinfecdis.7b00036 Nathaniel P. Endicott 1 , Eries Lee 1 , Timothy A. Wencewicz 1
Affiliation
|
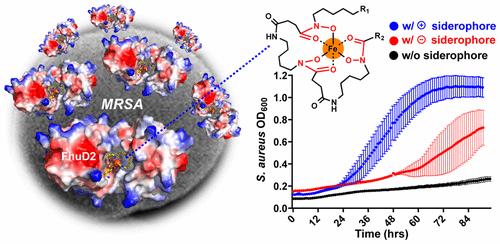
Staphylococcus aureus produces a cocktail of metallophores (staphylopine, staphyloferrin A, and staphyloferrin B) to scavenge transition metals during infection of a host. In addition, S. aureus displays the extracellular surface lipoproteins FhuD1 and FhuD2 along with the ABC transporter complex FhuCBG to facilitate the use of hydroxamate xenosiderophores such as desferrioxamine B (DFOB) for iron acquisition. DFOB is used as a chelation therapy to treat human iron overload diseases and has been linked to an increased risk of S. aureus infections. We used a panel of synthetic DFOB analogs and a FhuD2-selective trihydroxamate sideromycin to probe xenosiderophore utilization in S. aureus and establish structure–activity relationships for Fe(III) binding, FhuD2 binding, S. aureus growth promotion, and competition for S. aureus cell entry. Fe(III) binding assays and FhuD2 intrinsic fluorescence quenching experiments revealed that diverse chemical modifications of the terminal ends of linear ferrioxamine siderophores influences Fe(III) affinity but not FhuD2 binding. Siderophore–sideromycin competition assays and xenosiderophore growth promotion assays revealed that S. aureus SG511 and ATCC 11632 can distinguish between competing siderophores based exclusively on net charge of the siderophore–Fe(III) complex. Our work provides a roadmap for tuning hydroxamate xenosiderophore scaffolds to suppress (net negative charge) or enhance (net positive or neutral charge) uptake by S. aureus for applications in metal chelation therapy and siderophore-mediated antibiotic delivery, respectively.
中文翻译:

人类病原体金黄色葡萄球菌利用异种铁载体的结构基础
金黄色葡萄球菌可产生金属团的混合物(葡萄球菌素,葡萄球菌素A和葡萄球菌素B),以清除宿主感染期间的过渡金属。此外,金黄色葡萄球菌还展示了细胞外表面脂蛋白FhuD1和FhuD2以及ABC转运蛋白复合物FhuCBG,以促进使用异羟肟酸异种铁载体,如去铁胺B(DFOB)来获取铁。DFOB被用作螯合疗法来治疗人类铁超负荷疾病,并与金黄色葡萄球菌感染的风险增加有关。我们使用了一组合成的DFOB类似物和一个FhuD2选择性三氢异羟肟酸酯Sideromycin,以探究Xenosiderophore的利用金黄色葡萄球菌并建立与Fe(III)结合,FhuD2结合,金黄色葡萄球菌生长促进和金黄色葡萄球菌细胞进入竞争的结构-活性关系。Fe(III)结合测定和FhuD2内在的荧光猝灭实验表明,线性亚铁胺铁载体末端的多种化学修饰会影响Fe(III)亲和力,但不会影响FhuD2结合。铁载体-金霉素竞争试验和异铁载体生长促进试验表明金黄色葡萄球菌SG511和ATCC 11632可以仅根据铁载体-Fe(III)络合物的净电荷来区分竞争性铁载体。我们的工作提供了一个路线图,用于调整异羟肟酸酯异铁载体在金属螯合疗法和铁载体介导的抗生素应用中的抑制,以抑制(净负电荷)或增强(净正电荷或中性净电荷)对金黄色葡萄球菌的吸收。
更新日期:2017-05-30
中文翻译:

人类病原体金黄色葡萄球菌利用异种铁载体的结构基础
金黄色葡萄球菌可产生金属团的混合物(葡萄球菌素,葡萄球菌素A和葡萄球菌素B),以清除宿主感染期间的过渡金属。此外,金黄色葡萄球菌还展示了细胞外表面脂蛋白FhuD1和FhuD2以及ABC转运蛋白复合物FhuCBG,以促进使用异羟肟酸异种铁载体,如去铁胺B(DFOB)来获取铁。DFOB被用作螯合疗法来治疗人类铁超负荷疾病,并与金黄色葡萄球菌感染的风险增加有关。我们使用了一组合成的DFOB类似物和一个FhuD2选择性三氢异羟肟酸酯Sideromycin,以探究Xenosiderophore的利用金黄色葡萄球菌并建立与Fe(III)结合,FhuD2结合,金黄色葡萄球菌生长促进和金黄色葡萄球菌细胞进入竞争的结构-活性关系。Fe(III)结合测定和FhuD2内在的荧光猝灭实验表明,线性亚铁胺铁载体末端的多种化学修饰会影响Fe(III)亲和力,但不会影响FhuD2结合。铁载体-金霉素竞争试验和异铁载体生长促进试验表明金黄色葡萄球菌SG511和ATCC 11632可以仅根据铁载体-Fe(III)络合物的净电荷来区分竞争性铁载体。我们的工作提供了一个路线图,用于调整异羟肟酸酯异铁载体在金属螯合疗法和铁载体介导的抗生素应用中的抑制,以抑制(净负电荷)或增强(净正电荷或中性净电荷)对金黄色葡萄球菌的吸收。



























 京公网安备 11010802027423号
京公网安备 11010802027423号