当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photo-Uncaging by C(sp3)–C(sp3) Bond Cleavage Restores β-Lapachone Activity
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2023-06-02 , DOI: 10.1021/jacs.3c00398 Esther G Kaye 1, 2 , Bijan Mirabi 3 , Ivonne R Lopez-Miranda 1, 2 , Komadhie C Dissanayake 4 , Upasana Banerjee 4 , Madelyn Austin 4 , Mark Lautens 3 , Arthur H Winter 4 , Andrew A Beharry 1, 2
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2023-06-02 , DOI: 10.1021/jacs.3c00398 Esther G Kaye 1, 2 , Bijan Mirabi 3 , Ivonne R Lopez-Miranda 1, 2 , Komadhie C Dissanayake 4 , Upasana Banerjee 4 , Madelyn Austin 4 , Mark Lautens 3 , Arthur H Winter 4 , Andrew A Beharry 1, 2
Affiliation
|
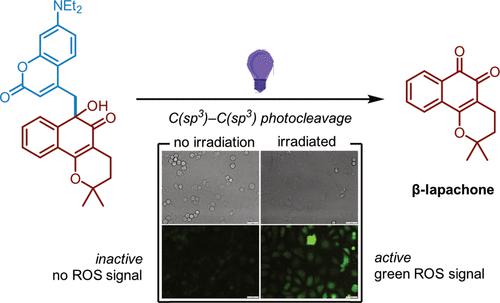
β-Lapachone is an ortho-naphthoquinone natural product with significant antiproliferative activity but suffers from adverse systemic toxicity. The use of photoremovable protecting groups to covalently inactivate a substrate and then enable controllable release with light in a spatiotemporal manner is an attractive prodrug strategy to limit toxicity. However, visible light-activatable photocages are nearly exclusively enabled by linkages to nucleophilic functional sites such as alcohols, amines, thiols, phosphates, and sulfonates. Herein, we report covalent inactivation of the electrophilic quinone moiety of β-lapachone via a C(sp3)–C(sp3) bond to a coumarin photocage. In contrast to β-lapachone, the designed prodrug remained intact in human whole blood and did not induce methemoglobinemia in the dark. Under light activation, the C–C bond cleaves to release the active quinone, recovering its biological activity when evaluated against the enzyme NQO1 and human cancer cells. Investigations into this report of a C(sp3)–C(sp3) photoinduced bond cleavage suggest a nontraditional, radical-based mechanism of release beginning with an initial charge-transfer excited state. Additionally, caging and release of the isomeric para-quinone, α-lapachone, are demonstrated. As such, we describe a photocaging strategy for the pair of quinones and report a unique light-induced cleavage of a C–C bond. We envision that this photocage strategy can be extended to quinones beyond β- and α-lapachone, thus expanding the chemical toolbox of photocaged compounds.
中文翻译:

通过 C(sp3)–C(sp3) 键断裂进行光解笼可恢复 β-Lapachone 活性
β-Lapachone 是一种邻萘醌天然产物,具有显着的抗增殖活性,但具有不良的全身毒性。使用光可去除的保护基团使底物共价失活,然后以时空方式利用光实现可控释放,是限制毒性的一种有吸引力的前药策略。然而,可见光激活的光笼几乎完全是通过与醇、胺、硫醇、磷酸盐和磺酸盐等亲核功能位点的连接来实现的。在此,我们报道了 β-lapachone 的亲电醌部分通过 C(sp 3 )–C(sp 3)与香豆素光笼键合。与β-拉帕酮相比,设计的前药在人全血中保持完整,并且在黑暗中不会诱发高铁血红蛋白血症。在光激活下,C-C 键断裂,释放出活性醌,在针对酶 NQO1 和人类癌细胞进行评估时恢复其生物活性。对 C(sp 3 )–C(sp 3 ) 光致键断裂报告的研究表明,存在一种非传统的、基于自由基的释放机制,从初始电荷转移激发态开始。此外,异构体的笼养和释放证明了-醌、α-拉帕酮。因此,我们描述了一对醌的光笼策略,并报告了独特的光诱导 C-C 键断裂。我们设想这种光笼策略可以扩展到β-和α-拉帕酮以外的醌类,从而扩展光笼化合物的化学工具箱。
更新日期:2023-06-02
中文翻译:

通过 C(sp3)–C(sp3) 键断裂进行光解笼可恢复 β-Lapachone 活性
β-Lapachone 是一种邻萘醌天然产物,具有显着的抗增殖活性,但具有不良的全身毒性。使用光可去除的保护基团使底物共价失活,然后以时空方式利用光实现可控释放,是限制毒性的一种有吸引力的前药策略。然而,可见光激活的光笼几乎完全是通过与醇、胺、硫醇、磷酸盐和磺酸盐等亲核功能位点的连接来实现的。在此,我们报道了 β-lapachone 的亲电醌部分通过 C(sp 3 )–C(sp 3)与香豆素光笼键合。与β-拉帕酮相比,设计的前药在人全血中保持完整,并且在黑暗中不会诱发高铁血红蛋白血症。在光激活下,C-C 键断裂,释放出活性醌,在针对酶 NQO1 和人类癌细胞进行评估时恢复其生物活性。对 C(sp 3 )–C(sp 3 ) 光致键断裂报告的研究表明,存在一种非传统的、基于自由基的释放机制,从初始电荷转移激发态开始。此外,异构体的笼养和释放证明了-醌、α-拉帕酮。因此,我们描述了一对醌的光笼策略,并报告了独特的光诱导 C-C 键断裂。我们设想这种光笼策略可以扩展到β-和α-拉帕酮以外的醌类,从而扩展光笼化合物的化学工具箱。


























 京公网安备 11010802027423号
京公网安备 11010802027423号