当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Microscopic-Level Insights into Solvation Chemistry for Nonsolvating Diluents Enabling High-Voltage/Rate Aqueous Supercapacitors
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2023-05-31 , DOI: 10.1021/jacs.3c02754 Jinhe Yu 1 , Chang Yu 1 , Xuedan Song 1 , Qing Zhang 1 , Zhao Wang 1 , Yuanyang Xie 1 , Yingbin Liu 1 , Wenbin Li 1 , Yiwang Ding 1 , Jieshan Qiu 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2023-05-31 , DOI: 10.1021/jacs.3c02754 Jinhe Yu 1 , Chang Yu 1 , Xuedan Song 1 , Qing Zhang 1 , Zhao Wang 1 , Yuanyang Xie 1 , Yingbin Liu 1 , Wenbin Li 1 , Yiwang Ding 1 , Jieshan Qiu 1
Affiliation
|
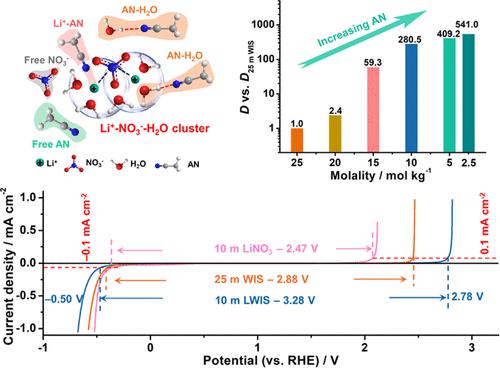
Localized “water-in-salt” (LWIS) electrolytes are promising candidates for the next generation of high-voltage aqueous electrolytes with low viscosity/salt beyond high-salt electrolytes. An effective yet high-function diluent mainly determines the properties of LWIS electrolytes, being a key issue. Herein, the donor number of solvents is identified to serve as a descriptor of interaction intensity between solvents and salts to screen the organic diluents having few impacts on the solvation microenvironment and intrinsic properties of the original high-salt electrolyte, further leading to the construction of a novel low-viscosity electrolyte with a low dosage of the LiNO3 salt and well-kept intrinsic Li+–NO3––H2O clusters. Nonsolvating diluents, especially acetonitrile (AN) that has never been reported previously, are presented with the capability of constructing a LWIS electrolyte with nonflammability, electrode-philic features, lower viscosity, decreased salt dosage, and a greatly enhanced ion diffusion coefficient by about 280 times. This strongly relies on a huge difference of about 5000 times in coordination and solubility between AN and H2O toward LiNO3 (0.05 vs 25 mol kgsolvent–1) and the moderate interaction between AN and H2O. Multi-spectroscopic techniques and molecular dynamics simulations uncover the solvation chemistry at the microscopic level and the interplay among cations, anions, and H2O without/with AN. The identified unique diluting and nonsolvating effects of AN reveal well-maintained cation–anion–H2O clusters and enhanced intermolecular hydrogen bonding between AN and H2O, further reinforcing the H2O stability and expanding the voltage window up to 3.28 V. This is a breakthrough that is far beyond high-viscosity/salt electrolytes for high-voltage and high-rate aqueous supercapacitors.
中文翻译:

对非溶剂稀释剂溶剂化化学的微观洞察,实现高电压/高倍率水性超级电容器
局部“盐包水”(LWIS)电解质是除高盐电解质之外具有低粘度/低盐的下一代高压水性电解质的有希望的候选者。有效且高性能的稀释剂主要决定LWIS电解质的性能,是一个关键问题。在此,确定了溶剂的供体数量作为溶剂和盐之间相互作用强度的描述符,以筛选对溶剂化微环境和原始高盐电解质的固有性质影响较小的有机稀释剂,进一步构建了一种新型低粘度电解质,具有低剂量的 LiNO 3盐和保持良好的固有 Li + –NO 3 – –H 2O 簇。非溶剂性稀释剂,特别是以前从未报道过的乙腈(AN),具有构建LWIS电解质的能力,该电解质具有不易燃性、亲电极特性、较低的粘度、减少的盐用量以及大大提高的离子扩散系数约280次。这很大程度上依赖于 AN 和 H 2 O 对 LiNO 3(0.05 vs 25 mol kg溶剂–1)之间的配位和溶解度存在约 5000 倍的巨大差异,以及 AN 和 H 2 O 之间的适度相互作用。分子动力学模拟揭示了微观层面的溶剂化化学以及阳离子、阴离子和 H 2之间的相互作用O 不带/带 AN。AN 独特的稀释和非溶剂化作用揭示了维持良好的阳离子-阴离子-H 2 O 簇以及 AN 和 H 2 O之间增强的分子间氢键,进一步增强了 H 2 O 的稳定性并将电压窗口扩大到 3.28 V。这是一项远远超出用于高电压和高速率水系超级电容器的高粘度/盐电解质的突破。
更新日期:2023-05-31
中文翻译:

对非溶剂稀释剂溶剂化化学的微观洞察,实现高电压/高倍率水性超级电容器
局部“盐包水”(LWIS)电解质是除高盐电解质之外具有低粘度/低盐的下一代高压水性电解质的有希望的候选者。有效且高性能的稀释剂主要决定LWIS电解质的性能,是一个关键问题。在此,确定了溶剂的供体数量作为溶剂和盐之间相互作用强度的描述符,以筛选对溶剂化微环境和原始高盐电解质的固有性质影响较小的有机稀释剂,进一步构建了一种新型低粘度电解质,具有低剂量的 LiNO 3盐和保持良好的固有 Li + –NO 3 – –H 2O 簇。非溶剂性稀释剂,特别是以前从未报道过的乙腈(AN),具有构建LWIS电解质的能力,该电解质具有不易燃性、亲电极特性、较低的粘度、减少的盐用量以及大大提高的离子扩散系数约280次。这很大程度上依赖于 AN 和 H 2 O 对 LiNO 3(0.05 vs 25 mol kg溶剂–1)之间的配位和溶解度存在约 5000 倍的巨大差异,以及 AN 和 H 2 O 之间的适度相互作用。分子动力学模拟揭示了微观层面的溶剂化化学以及阳离子、阴离子和 H 2之间的相互作用O 不带/带 AN。AN 独特的稀释和非溶剂化作用揭示了维持良好的阳离子-阴离子-H 2 O 簇以及 AN 和 H 2 O之间增强的分子间氢键,进一步增强了 H 2 O 的稳定性并将电压窗口扩大到 3.28 V。这是一项远远超出用于高电压和高速率水系超级电容器的高粘度/盐电解质的突破。



























 京公网安备 11010802027423号
京公网安备 11010802027423号