RSC主编推荐:能源领域精彩文章快览(免费阅读原文)
英国皇家化学会(RSC)是一个拥有175年历史的面向全球化学家的非营利会员制机构,旗下拥有43种期刊,其中很多在化学领域有很高影响力。为了进一步帮助广大读者追踪科技前沿热点,X-MOL团队与英国皇家化学会合作,推出英国皇家化学会期刊主编推荐的精彩文章快览,本期文章属“能源领域”,英文点评来自英国皇家化学会期刊的主编。如果大家对我们的解读有更多的补充和点评,欢迎在文末写评论发表您的高见!
Energy & Environmental Science
(IF: 25.43 )

1. Efficient Catalytic Greenhouse Gas-Free Hydrogen and Aldehyde Formation from Aqueous Alcohol Solutions
Energy Environ. Sci., 2017, Advance Article
DOI: 10.1039/C6EE03739A
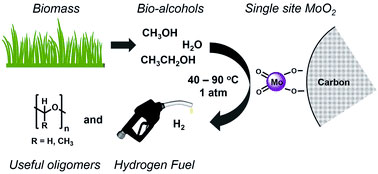
Hydrogen is an ideal fuel candidate due to its clean combustion characteristics. Here the authors report an inexpensive and sustainable Mo-based, carbon-supported catalyst that generates H2 and the corresponding aldehyde from aqueous methanol or ethanol at high rates with negligible greenhouse gas co-production. The aldehyde coproducts lend value to this process without producing CO2.
由于其清洁燃烧特性,氢气被认为是理想燃料的候选。本文作者报道了一种低成本且可持续的碳负载的钼基催化剂,这种催化剂能以高速率从甲醇或乙醇的水溶液出发催化产生氢气和相对应的醛,并且几乎无温室气体产生。共产物醛使得这一不产生CO2的反应极具价值。
限时免费阅读原文,登陆后可下载
扫描或长按二维码,识别后直达原文页面,或点此查看原文

2. In vivo activation of an [FeFe] hydrogenase using synthetic cofactors
Energy Environ. Sci., 2017, Advance Article
DOI: 10.1039/C7EE00135E
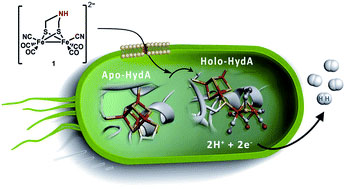
[FeFe] hydrogenases catalyze the reduction of protons, and oxidation of hydrogen gas, with remarkable efficiency. The reaction occurs at the H-cluster, which contains an organometallic [2Fe] subsite. The unique nature of the [2Fe] subsite makes it dependent on a specific set of maturation enzymes for its biosynthesis and incorporation into the apo-enzyme. The authors report on how this can be circumvented, and the apo-enzyme activated in vivo by synthetic active site analogues taken up by the living cell.
[FeFe]氢化酶能以很高的效率催化质子还原和氢气氧化。反应发生在含有有机金属[2Fe]亚位点的氢簇上。[2Fe]亚位点的独特性质使得其在生物合成和结合酶蛋白的过程中依赖于特定的成熟酶。本文作者报道了如何避免这种情况,他们采用的是合成的活性位点类似物,当其被活细胞摄取后,酶蛋白在体内被成功激活。
限时免费阅读原文,登陆后可下载
扫描或长按二维码,识别后直达原文页面,或点此查看原文

3. Compatibility issues between electrodes and electrolytes in solid-state batteries
Energy Environ. Sci., 2017, Advance Article
DOI: 10.1039/C7EE00534B

Remarkable success has been achieved in the discovery of ceramic alkali superionic conductors as electrolytes in solid-state batteries; however, obtaining a stable interface between these electrolytes and electrodes is difficult. Only limited studies on the compatibility between electrodes and solid electrolytes have been reported, partially because of the need for expensive instrumentation and special cell designs. Without simple yet powerful tools, these compatibility issues cannot be systematically investigated, thus hindering the generalization of design rules for the integration of solid-state battery components.
The authors present a methodology that combines density functional theory calculations and simple experimental techniques such as X-ray diffraction, simultaneous differential scanning calorimetry and thermal gravimetric analysis, and electrochemistry to efficiently screen the compatibility of numerous electrode /electrolyte pairs. They systemically distinguish between the electrochemical stability of the solid-state conductor, which is relevant wherever the electrolyte contacts an electron pathway, and the electrochemical stability of the electrode/electrolyte interfaces. For the solid electrolyte, the authors are able to computationally derive an absolute thermodynamic stability voltage window, which is small for Na3PS4 and Na3PSe4, and a larger voltage window which can be kinetically stabilized. The experimental stability, when measured with reliable techniques, falls between these thermodynamic and kinetic limits. Employing a Na solid-state system as an example, they demonstrate the efficiency of the method by finding the most stable system (NaCrO2|Na3PS4|Na–Sn) within a selected chemical space (more than 20 different combinations of electrodes and electrolytes). Important selection criteria for the cathode, electrolyte, and anode in solid-state batteries are also derived from this study.
The current method not only provides an essential guide for integrating all-solid-state battery components but can also significantly accelerate the expansion of the electrolyte/electrode compatibility data.
在固态电池中作为电解质的陶瓷碱性超离子导体的研究已经取得了显著的进展。但是,在电解质和电极之间获得稳定的界面是十分困难的。只有少数针对电极和固体电解质兼容性的研究有所报道,部分原因是这需要昂贵的仪器和特殊的电池设计。没有简单但强大的工具支持,兼容性的问题难以得到系统研究,因而也阻碍了固态电池组件集成设计规则的归纳。
本文作者介绍了一种方法,结合密度泛函理论计算和简单的实验技术(如X射线衍射法、同时差示扫描量热法、热重分析法、电化学法)以高效筛选大量电解质和电极对的兼容性。他们系统地区分了与电解质接触电子通路相关的固态导体的电化学稳定性和电极-电解液界面的电化学稳定性。对于固体电解质,作者可以通过计算得出绝对热力学稳定电压窗口,其对于Na3PS4和Na3PSe4来说很小,以及一个可动力学稳定的较大的电压窗口。通过可靠技术测量得到的实验稳定性在热力学和动力学限制之间。以钠固态体系为例,他们用这种方法在给定的化学空间(超过二十组电解质与电极的组合)中挑选出了最稳定的体系(NaCrO2|Na3PS4|Na–Sn),从而展示了其高效性。固态电池中的正极、电解质以及负极的重要选取标准也在这项研究中得到阐述。
这一方法不仅为全固态电池组件集成提供了重要的指导,而且也加速了电解质和电极兼容数据的扩展。
限时免费阅读原文,登陆后可下载
扫描或长按二维码,识别后直达原文页面,或点此查看原文

Chemical Science
(IF: 9.144)

1. Highly efficient electrochemical reduction of CO2 to CH4 in an ionic liquid using a metal-organic framework cathode
Chem. Sci., 2016, 7, 890-895
DOI: 10.1039/ C5SC03291A
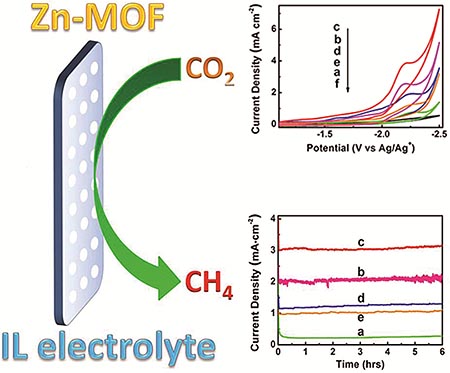
Researchers in Beijing have reported an efficient electrochemical reduction of CO2 to CH4. This was achieved via the combination of a metal-organic framework cathode and a pure ionic liquid electrolyte. Compared to commonly used metal electrodes, the reaction exhibited a higher selectivity to CH4 and current density, and the overpotential for CH4 was lower. This new combination provides a method to effectively and selectively reduce CO2 to CH4.
中科院化学所的研究人员报道了一种电化学还原法,通过结合金属有机骨架(MOF)阴极和纯离子液体电解质可高效地将二氧化碳(CO2)转化为甲烷(CH4)。与常用的金属电极相比,这种方法对甲烷和电流密度展现出更高的选择性,而甲烷的过电位更低。这一新组合为二氧化碳转化成甲烷提供了一种高效和高选择性的方法。
Open Access(可免费阅读原文)
扫描或长按二维码,识别后直达原文页面,或点此查看原文

2. Noble-Metal-Free Co3S4-S/G Porous Hybrids As an Efficient Electrocatalyst for Oxygen Reduction Reaction
Chem. Sci., 2016, 7, 6721-6727
DOI: 10.1039/C6SC00357E
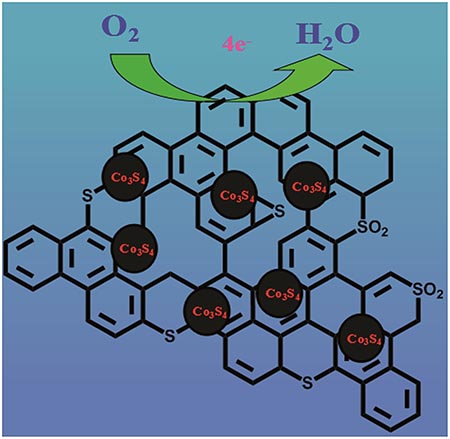
Chinese scientists have developed an efficient electrocatalyst for the oxygen reduction reaction. The noble-metal-free catalyst consisted of CO3S4 nanoparticles encapsulated in porous sulfur doped graphene. The catalytic activity was comparable with commercially available platinum catalysts, and displayed higher stability. These findings are promising for the future production of an efficient, stable and low-cost catalyst.
中科院长春应化所的研究人员开发了一种氧还原反应(ORR)的高效电催化剂。该催化剂不含贵金属,由包裹于多孔硫掺杂的石墨烯中的CO3S4纳米颗粒组成,催化活性与市售的铂催化剂相当,但展现了更高的稳定性。这一成果有望推动未来高效、稳定以及低成本ORR催化剂的生产。
Open Access(可免费阅读原文)
扫描或长按二维码,识别后直达原文页面,或点此查看原文

如果篇首注明了授权来源,任何转载需获得来源方的许可!如果篇首未特别注明出处,本文版权属于 X-MOL ( x-mol.com ), 未经许可,谢绝转载!




























 京公网安备 11010802027423号
京公网安备 11010802027423号