RSC主编推荐:有机领域精彩文章快览(免费阅读原文)
英国皇家化学会(RSC)是一个拥有超过175年历史的面向全球化学家的非营利会员制机构,旗下拥有44种期刊,其中很多在化学领域有很高影响力。为了进一步帮助广大读者追踪科技前沿热点,X-MOL团队与英国皇家化学会合作,推出英国皇家化学会期刊主编推荐的精彩文章快览,本期文章属“有机领域”,英文点评来自英国皇家化学会期刊的主编。如果大家对我们的解读有更多的补充和点评,欢迎在文末写评论发表您的高见!
Chemical Science (IF: 9.063)

1. Rhodium-catalyzed ortho-heteroarylation of phenols: directing group-enabled switching of the electronic bias for heteroaromatic coupling partner
Chem. Sci., 2018, Advance Article
DOI: 10.1039/C8SC02529K
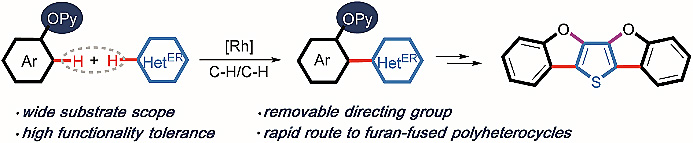
Researchers at Sichuan University are reporting on Rh(III)-catalysed ortho-heteroarylation of phenols with electron-rich heteroarenes via two-fold C-H activation. Directed oxidative C-H/C-H cross coupling reactions involving functionalised arenes and heteroarenes normally show an electronic bias for the heteroaromatic coupling partner. The authors have now presented a strategy that demonstrates directing-group enabled switching of the electronic bias for the coupling partner from the electron-deficient to electron-rich heteroarene.
四川大学的研究人员报道了Rh(III)催化酚类化合物与富电子杂芳香烃通过双重C-H键活化进行的邻位杂芳基化反应。定向氧化的C-H/C-H键交叉偶联反应涉及官能化的芳香烃和杂芳香烃,通常对不同杂芳基偶联体表现出电子偏好。本文作者们提出了一种策略,表明导向基团能够将偶联体的电子偏好从缺电子的杂芳香烃转为富电子的杂芳香烃。
Open Access(可免费阅读原文)
扫描或长按二维码,识别后直达原文页面,或点此查看原文

2. Nickel(0)-catalyzed linear-selective hydroarylation of unactivated alkenes and styrenes with aryl boronic acids
Chem. Sci., 2018, Advance Article
DOI: 10.1039/C8SC02101E
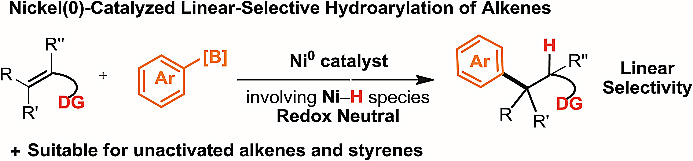
Researchers at Nankai University have developed the first method for directing-group controlled linear-selective hydroarylation of unactivated alkenes and styrenes with aryl boronic acids. This method enables the preparation of structurally diverse alkyl arenes, including γ-aryl butyric acid derivatives which can be utilised for the preparation of biologically active compounds.
南开大学的研究人员首次发展了导向基团控制的非活化烯烃及苯乙烯与芳基硼酸的线性选择性氢芳基化反应。该方法可用于制备结构多样的烷基芳香烃,包括可用于制备生物活性化合物的γ-芳基丁酸衍生物。
Open Access(可免费阅读原文)
扫描或长按二维码,识别后直达原文页面,或点此查看原文

Organic Chemistry Frontiers (IF: 5.455)

1. A facile synthesis of diverse 5-arylated triazoles via a Cu-catalyzed oxidative interrupted click reaction with arylboronic acids in air
Org. Chem. Front., 2018, Advance Article
DOI: 10.1039/C8QO00590G
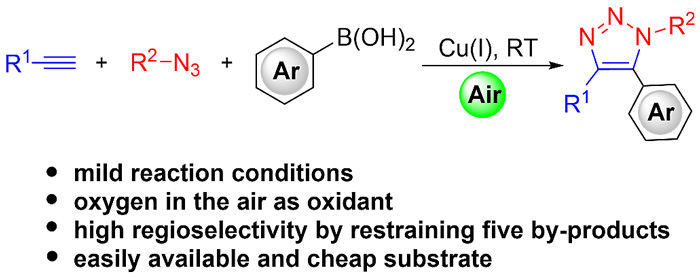
The authors reported a Cu-catalyzed synthesis of 5-arylsubstituted 1,2,3-triazoles via an oxidative interrupted click reaction, using air as the sole terminal oxidant. The current protocol provides a novel method for the one-step construction of fully substituted triazoles in good yields under mild conditions, in which arylboronic acids are employed as aromatic sources to interrupt the cuprate–triazole intermediate for the first time.
作者报道了一种5-芳基取代1,2,3-三唑的Cu催化合成方法,该方法借助氧化阻断的点击反应,并使用空气作为唯一的终止氧化剂。这种新颖的方法首次将芳基硼酸作为芳基来源,用于阻断铜酸盐-三唑中间体,能够以良好的产率、温和的条件一步合成全取代的三唑化合物。
限时免费阅读原文,登陆后可下载
扫描或长按二维码,识别后直达原文页面,或点此查看原文

2. Green carbon disulfide surrogate via a combination of potassium sulfide and chloroform for benzothiazine-thione and benzothiazole-thione constructions
Org. Chem. Front., 2018, Advance Article
DOI: 10.1039/C8QO00481A
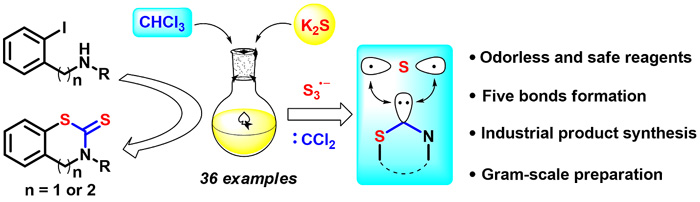
An efficient and green carbon disulfide surrogate via facile combination of potassium sulfide and chloroform has been developed. A variety of benzothiazine-thiones and benzothiazole-thiones were straight forwardly established along with the formation of five new chemical bonds in one pot from readily available starting materials, in which the widely used 2-mercaptobenzothiazole (MBT) was synthesized through this method in gram scale.
研究人员通过硫化钾和氯仿的简单结合,开发出一种高效且绿色的二硫化碳替代物。他们使用易获取的原料,以一锅法直接合成了多种苯并噻嗪-硫酮和苯并噻唑-硫酮,同时形成五个新的化学键。该方法可用于克量级规模合成应用广泛的2-巯基苯并噻唑(MBT)。
限时免费阅读原文,登陆后可下载
扫描或长按二维码,识别后直达原文页面,或点此查看原文

如果篇首注明了授权来源,任何转载需获得来源方的许可!如果篇首未特别注明出处,本文版权属于 X-MOL ( x-mol.com ), 未经许可,谢绝转载!



























 京公网安备 11010802027423号
京公网安备 11010802027423号