Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemical modification of antibodies enables the formation of stable antibody–gold nanoparticle conjugates for biosensing
Analyst ( IF 4.2 ) Pub Date : 2017-10-26 00:00:00 , DOI: 10.1039/c7an01496a Seth L. Filbrun 1, 2, 3, 4 , Alexandra B. Filbrun 1, 2, 3, 4 , Francis L. Lovato 1, 2, 3, 4 , Soon H. Oh 4, 5, 6, 7 , Elizabeth A. Driskell 4, 5, 6, 7 , Jeremy D. Driskell 1, 2, 3, 4
Analyst ( IF 4.2 ) Pub Date : 2017-10-26 00:00:00 , DOI: 10.1039/c7an01496a Seth L. Filbrun 1, 2, 3, 4 , Alexandra B. Filbrun 1, 2, 3, 4 , Francis L. Lovato 1, 2, 3, 4 , Soon H. Oh 4, 5, 6, 7 , Elizabeth A. Driskell 4, 5, 6, 7 , Jeremy D. Driskell 1, 2, 3, 4
Affiliation
|
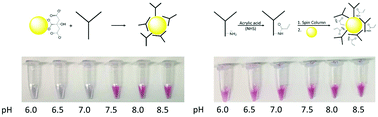
Antibody-modified gold nanoparticles (AuNPs) are central to many novel and emerging biosensing technologies due to the specificity provided by antibody–antigen interactions and the unique properties of nanoparticles. These AuNP-enabled assays have the potential to provide significant improvements in sensitivity and multiplexed analysis compared to conventional immunoassays. However, a major challenge for these AuNP platform technologies is the synthesis of stable antibody–AuNP conjugates that resist aggregation in high salt environments and biological matrices. Moreover, synthetic strategies to form stable conjugates often require different solution conditions, e.g., pH, for each unique antibody. Herein we describe our effort to develop an approach to chemically modify lysine residues on antibodies to facilitate the formation of stable antibody–AuNP conjugates over a wide pH range. In this work, we systematically investigated the immobilization of native and chemically modified antibodies to 60 nm citrate-capped AuNPs as a function of pH and evaluated the stability of the antibody–AuNP conjugate in a saline environment. We have developed a method to chemically modify the lysine residues on an antibody prior to conjugation to the AuNP that results in stable conjugates over a wide pH range (6.0–8.5). Amino acid analysis and zeta potential measurements of native and modified antibodies reveal that the requisite modification correlates with the number of lysine residues, and a reduction in positive charge contribution from protonated lysine is required to form stable, pH-independent conjugates. Furthermore, we demonstrate that the chemically modified antibodies maintain antigen-binding capabilities. We apply this novel conjugation strategy to develop a surface-enhanced Raman spectroscopy (SERS)-based assay for the accurate subtyping of avian influenza viruses.
中文翻译:

抗体的化学修饰可形成稳定的抗体-金纳米颗粒偶联物,用于生物传感
抗体修饰的金纳米颗粒(AuNPs)由于抗体-抗原相互作用提供的特异性以及纳米颗粒的独特特性,在许多新型和新兴的生物传感技术中至关重要。与传统的免疫测定相比,这些启用AuNP的测定具有显着提高灵敏度和多重分析的潜力。然而,这些AuNP平台技术的主要挑战是合成稳定的抗体-AuNP结合物,该结合物可抵抗高盐环境和生物基质中的聚集。此外,形成稳定缀合物的合成策略通常需要不同的溶液条件,例如每种独特抗体的pH值。本文中,我们描述了我们的工作,以开发一种化学修饰抗体上赖氨酸残基的方法,以促进在较宽的pH范围内形成稳定的抗体-AuNP偶联物。在这项工作中,我们系统地研究了天然和化学修饰的抗体对60 nm柠檬酸盐封端的AuNP的固定与pH的函数关系,并评估了在盐水环境中抗体-AuNP缀合物的稳定性。我们已经开发了一种化学修饰抗体上赖氨酸残基的方法,使其与AuNP结合,从而可以在很宽的pH范围(6.0-8.5)范围内产生稳定的结合物。天然抗体和修饰抗体的氨基酸分析和Zeta电位测量结果表明,必需的修饰与赖氨酸残基的数量有关,并需要降低质子化赖氨酸的正电荷贡献,以形成稳定的,pH无关的结合物。此外,我们证明化学修饰的抗体保持抗原结合能力。我们应用这种新颖的共轭策略来开发基于表面增强拉曼光谱(SERS)的测定方法,用于禽流感病毒的精确亚型分析。
更新日期:2017-11-20
中文翻译:

抗体的化学修饰可形成稳定的抗体-金纳米颗粒偶联物,用于生物传感
抗体修饰的金纳米颗粒(AuNPs)由于抗体-抗原相互作用提供的特异性以及纳米颗粒的独特特性,在许多新型和新兴的生物传感技术中至关重要。与传统的免疫测定相比,这些启用AuNP的测定具有显着提高灵敏度和多重分析的潜力。然而,这些AuNP平台技术的主要挑战是合成稳定的抗体-AuNP结合物,该结合物可抵抗高盐环境和生物基质中的聚集。此外,形成稳定缀合物的合成策略通常需要不同的溶液条件,例如每种独特抗体的pH值。本文中,我们描述了我们的工作,以开发一种化学修饰抗体上赖氨酸残基的方法,以促进在较宽的pH范围内形成稳定的抗体-AuNP偶联物。在这项工作中,我们系统地研究了天然和化学修饰的抗体对60 nm柠檬酸盐封端的AuNP的固定与pH的函数关系,并评估了在盐水环境中抗体-AuNP缀合物的稳定性。我们已经开发了一种化学修饰抗体上赖氨酸残基的方法,使其与AuNP结合,从而可以在很宽的pH范围(6.0-8.5)范围内产生稳定的结合物。天然抗体和修饰抗体的氨基酸分析和Zeta电位测量结果表明,必需的修饰与赖氨酸残基的数量有关,并需要降低质子化赖氨酸的正电荷贡献,以形成稳定的,pH无关的结合物。此外,我们证明化学修饰的抗体保持抗原结合能力。我们应用这种新颖的共轭策略来开发基于表面增强拉曼光谱(SERS)的测定方法,用于禽流感病毒的精确亚型分析。


























 京公网安备 11010802027423号
京公网安备 11010802027423号