当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Investigation of chloride ion adsorption onto Ti2C MXene monolayers by first-principles calculations
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2017-11-06 00:00:00 , DOI: 10.1039/c7ta09057a Dashuai Wang 1, 2, 3, 4, 5 , Yu Gao 1, 2, 3, 4, 5 , Yanhui Liu 5, 6, 7, 8, 9 , Yury Gogotsi 1, 2, 3, 4, 5 , Xing Meng 1, 2, 3, 4, 5 , Gang Chen 1, 2, 3, 4, 5 , Yingjin Wei 1, 2, 3, 4, 5
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2017-11-06 00:00:00 , DOI: 10.1039/c7ta09057a Dashuai Wang 1, 2, 3, 4, 5 , Yu Gao 1, 2, 3, 4, 5 , Yanhui Liu 5, 6, 7, 8, 9 , Yury Gogotsi 1, 2, 3, 4, 5 , Xing Meng 1, 2, 3, 4, 5 , Gang Chen 1, 2, 3, 4, 5 , Yingjin Wei 1, 2, 3, 4, 5
Affiliation
|
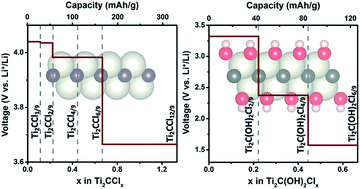
Chloride ion adsorption on Ti2C monolayers was theoretically investigated. Electrochemical parameters, including specific capacity, chloride ion (Cl−) diffusion barrier, and discharge voltage profile, were studied via first-principles calculations. The most favorable Cl− adsorption configuration was identified using a partial particle swarm optimization algorithm and the results showed that Cl− adsorption onto Ti2C monolayers achieved a large theoretical capacity (331 mA h g−1), high working voltage (4.0–3.5 V), and low diffusion barrier (0.22 eV). This resulted in excellent rate capability and a large specific energy of 1269 W h kg−1 at the material level. The effects of terminal O, F, and OH groups on Cl− adsorption onto Ti2C monolayer were also studied, which showed that Cl− could not be adsorbed onto O and F terminated Ti2C monolayers. In comparison, Cl− adsorption onto OH terminated Ti2C was allowed but generated a smaller specific capacity (126 mA h g−1) and lower working voltage (3.5–1.5 V) than a bare Ti2C monolayer.
中文翻译:

通过第一性原理研究氯离子在Ti 2 C MXene单层上的吸附
从理论上研究了氯离子在Ti 2 C单层上的吸附。电化学参数,包括具体的容量,氯离子(CL - )扩散阻挡层,并且放电电压分布进行了研究通过第一原理计算。最有利的氯-吸附构型使用部分粒子群优化算法鉴定,结果表明,氯-吸附的Ti 2个ç单层实现大的理论容量(331毫安汞柱-1),高工作电压(4.0-3.5 V )和低扩散势垒(0.22 eV)。这导致了出色的速率能力和1269 W h kg -1的大比能在物质层面上。端子O的影响,Cl为F和OH基团-吸附的Ti 2 ç单层也进行了研究,表明氯-不能被吸附到ö和F终止的Ti 2个Ç单层。相比之下,氯-吸附到OH封端的Ti 2下允许的,但所产生的较小的比容量(126毫安汞柱-1)以上且低于裸钛工作电压(3.5-1.5 V)2 Ç单层。
更新日期:2017-11-20
中文翻译:

通过第一性原理研究氯离子在Ti 2 C MXene单层上的吸附
从理论上研究了氯离子在Ti 2 C单层上的吸附。电化学参数,包括具体的容量,氯离子(CL - )扩散阻挡层,并且放电电压分布进行了研究通过第一原理计算。最有利的氯-吸附构型使用部分粒子群优化算法鉴定,结果表明,氯-吸附的Ti 2个ç单层实现大的理论容量(331毫安汞柱-1),高工作电压(4.0-3.5 V )和低扩散势垒(0.22 eV)。这导致了出色的速率能力和1269 W h kg -1的大比能在物质层面上。端子O的影响,Cl为F和OH基团-吸附的Ti 2 ç单层也进行了研究,表明氯-不能被吸附到ö和F终止的Ti 2个Ç单层。相比之下,氯-吸附到OH封端的Ti 2下允许的,但所产生的较小的比容量(126毫安汞柱-1)以上且低于裸钛工作电压(3.5-1.5 V)2 Ç单层。



























 京公网安备 11010802027423号
京公网安备 11010802027423号