当前位置:
X-MOL 学术
›
Cell Metab.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
AMPK: Sensing Glucose as well as Cellular Energy Status.
Cell Metabolism ( IF 29.0 ) Pub Date : 2017-11-16 , DOI: 10.1016/j.cmet.2017.10.009 Sheng-Cai Lin 1 , D Grahame Hardie 2
Cell Metabolism ( IF 29.0 ) Pub Date : 2017-11-16 , DOI: 10.1016/j.cmet.2017.10.009 Sheng-Cai Lin 1 , D Grahame Hardie 2
Affiliation
|
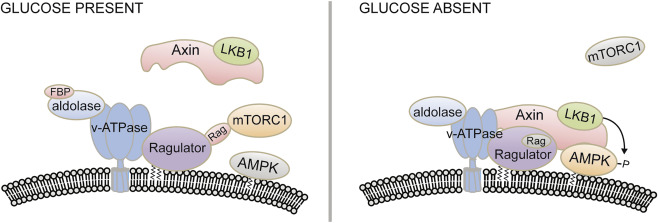
Mammalian AMPK is known to be activated by falling cellular energy status, signaled by rising AMP/ATP and ADP/ATP ratios. We review recent information about how this occurs but also discuss new studies suggesting that AMPK is able to sense glucose availability independently of changes in adenine nucleotides. The glycolytic intermediate fructose-1,6-bisphosphate (FBP) is sensed by aldolase, which binds to the v-ATPase on the lysosomal surface. In the absence of FBP, interactions between aldolase and the v-ATPase are altered, allowing formation of an AXIN-based AMPK-activation complex containing the v-ATPase, Ragulator, AXIN, LKB1, and AMPK, causing increased Thr172 phosphorylation and AMPK activation. This nutrient-sensing mechanism activates AMPK but also primes it for further activation if cellular energy status subsequently falls. Glucose sensing at the lysosome, in which AMPK and other components of the activation complex act antagonistically with another key nutrient sensor, mTORC1, may have been one of the ancestral roles of AMPK.
中文翻译:

AMPK:感应葡萄糖以及细胞能量状态。
众所周知,AMPAMP可以通过降低细胞的能量状态来激活,这可以通过提高AMP / ATP和ADP / ATP比率来实现。我们回顾了有关这种情况发生的最新信息,但也讨论了新的研究,这些研究表明AMPK能够独立于腺嘌呤核苷酸的变化而感知葡萄糖的可用性。糖酵解中间体1,6-二磷酸果糖(FBP)由醛缩酶检测,该醛缩酶与溶酶体表面的v-ATPase结合。在没有FBP的情况下,醛缩酶和v-ATPase之间的相互作用发生了改变,从而形成了基于AXIN的AMPK激活复合物,其中包含v-ATPase,Ragulator,AXIN,LKB1和AMPK,从而导致Thr172磷酸化和AMPK激活增加。如果细胞能量状态随后下降,这种营养传感机制既可以激活AMPK,又可以引发AMPK进一步激活。
更新日期:2018-02-02
中文翻译:

AMPK:感应葡萄糖以及细胞能量状态。
众所周知,AMPAMP可以通过降低细胞的能量状态来激活,这可以通过提高AMP / ATP和ADP / ATP比率来实现。我们回顾了有关这种情况发生的最新信息,但也讨论了新的研究,这些研究表明AMPK能够独立于腺嘌呤核苷酸的变化而感知葡萄糖的可用性。糖酵解中间体1,6-二磷酸果糖(FBP)由醛缩酶检测,该醛缩酶与溶酶体表面的v-ATPase结合。在没有FBP的情况下,醛缩酶和v-ATPase之间的相互作用发生了改变,从而形成了基于AXIN的AMPK激活复合物,其中包含v-ATPase,Ragulator,AXIN,LKB1和AMPK,从而导致Thr172磷酸化和AMPK激活增加。如果细胞能量状态随后下降,这种营养传感机制既可以激活AMPK,又可以引发AMPK进一步激活。


























 京公网安备 11010802027423号
京公网安备 11010802027423号